Abstract
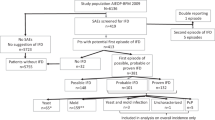
Studies that analyze the epidemiology and risk factors for invasive fungal disease (IFD) after engraftment in alloSCT are few in number. This single-center retrospective study included 404 alloSCT adult recipients surviving >40 days who engrafted and were discharged without prior IFD. All patients who received ⩾20 mg/day of prednisone were assigned to primary oral prophylaxis (itraconazole or low-dose voriconazole). The primary end point was the cumulative incidence (CI) of probable/proven IFD using the European Organization for Research and Treatment of Cancer and Mycoses Study Group (EORTC/MSG) criteria. The independent prognostic factors after multivariate analyses were used to construct a post-engraftment IFD risk score. The 1-year CI of IFD was 11%. The non-relapse mortality was 40% in those developing IFD and 16% in those who did not. The intent-to-treat analysis showed that 17% of patients abandoned the assigned prophylaxis. Age >40 years, ⩾1 previous SCT, pre-engraftment neutropenia >15 days, extensive chronic GVHD and CMV reactivation were independent risk factors. The post-engraftment IFD score stratified patients into low risk (0–1 factor, CI 0.7%), intermediate risk (2 factors, CI 9.9%) and high risk (3–5 factors, CI 24.7%) (P<0.0001). The antifungal prophylaxis strategy failed to prevent post-engraftment IFD in 11% of alloSCT. Our risk score could be useful to implement risk-adapted strategies using antifungal prophylaxis after engraftment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Zhang P, Jiang EL, Yang DL, Yan ZS, Huang Y, Wei JL et al. Risk factors and prognosis of invasive fungal infections in allogeneic stem cell transplantation recipients: a single-institution experience. Transpl Infect Dis 2010; 12: 316–321.
Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 2010; 50: 1091–1100.
Marr KA, Carter RA, Boeckh M, Martin P, Corey L . Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 2002; 100: 4358–4366.
Garcia-Vidal C, Upton A, Kirby KA, Marr KA . Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis 2008; 47: 1041–1050.
Mikulska M, Raiola AM, Bruno B, Furfaro E, Van Lint MT, Bregante S et al. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant 2009; 44: 361–370.
Marks DI, Pagliuca A, Kibbler CC, Glasmacher A, Heussel CP, Kantecki M et alIMPROVIT Study Group. Voriconazole versus itraconazole for antifungal prophylaxis following allogeneic haematopoietic stem-cell transplantation. Br J Haematol 2011; 155: 318–327.
Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 2007; 356: 335–347.
Winston DJ, Maziarz RT, Chandrasekar PH, Lazarus HM, Goldman M, Blumer JL et al. Intravenous and oral itraconazole versus intravenous and oral fluconazole for long-term antifungal prophylaxis in allogeneic hematopoietic stem-cell transplant recipients. A multicenter, randomized trial. Ann Intern Med 2003; 138: 705–713.
Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Baden LR et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 2010; 116: 5111–5118.
Martin T, Sharma M, Damon L, Kaplan L, Guglielmo BJ, Working M et al. Voriconazole is safe and effective as prophylaxis for early and late fungal infections following allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 2010; 12: 45–50.
Thursky K, Byrnes G, Grigg A, Szer J, Slavin M . Risk factors for post-engraftment invasive aspergillosis in allogeneic stem cell transplantation. Bone Marrow Transplant 2004; 34: 115–121.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Montesinos P, Sanz J, Cantero S, Lorenzo I, Martín G, Saavedra S et al. Incidence, risk factors, and outcome of cytomegalovirus infection and disease in patients receiving prophylaxis with oral valganciclovir or intravenous ganciclovir after umbilical cord blood transplantation. Biol Blood Marrow Transplant 2009; 15: 730–740.
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T et al. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46: 1813–1821.
Gray RJ . A class of K-sample test for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154.
Fine J, Gray R . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 2009; 48: 265–273.
Garcia-Vidal C, Royo-Cebrecos C, Peghin M, Moreno A, Ruiz-Camps I, Cervera C et al. Environmental variables associated with an increased risk of invasive aspergillosis. Clin Microbiol Infect 2014; 20: 0939–0945.
Siwek GT, Pfaller MA, Polgreen PM, Cobb S, Hoth P, Magalheas-Silverman M et al. Incidence of invasive aspergillosis among allogeneic hematopoietic stem cell transplant patients receiving voriconazole prophylaxis. Diagn Microbiol Infect Dis 2006; 55: 209–212.
El Cheikh J, Castagna L, Wang L, Esterni B, Faucher C, Furst S et al. Once-weekly liposomal amphotericin B for prophylaxis of invasive fungal infection after graft-versus-host disease in allogeneic hematopoietic stem cell transplantation: a comparative retrospective single-center study. Hematol Oncol Stem Cell Ther 2010; 3: 167–173.
Boogaerts MA, Verhoef GE, Zachee P, Demuynck H, Verbist L, De Beule K . Antifungal prophylaxis with itraconazole in prolonged neutropenia: correlation with plasma levels. Mycoses 1989; 32: 103–108.
Howard A, Hoffman J, Sheth A . Clinical application of voriconazole concentrations in the treatment of invasive aspergillosis. Ann Pharmacother 2008; 42: 1859–1864.
Hol JA, Wolfs TF, Bierings MB, Lindemans CA, Versluys AB, Wildt de A et al. Predictors of invasive fungal infection in pediatric allogeneic hematopoietic SCT recipients. Bone Marrow Transplant 2014; 49: 95–101.
Blennow O, Remberger M, Klingspor L, Omazic B, Fransson K, Ljungman P et al. Randomized PCR-based therapy and risk factors for invasive fungal infection following reduced-intensity conditioning and hematopoietic SCT. Bone Marrow Transplant 2010; 45: 1710–1718.
Acknowledgements
We thank Paula Petruskevicius, Carlos Pastorini, David Pellicer and Shirley Weiss for data collection and management. This study was in part supported by grant 2012/023 from the Instituto de Investigación Sanitaria La Fe. This paper was supported by an independent medical grant provided by Pfizer, Inc.
Author contributions
PM, RR-V and MAS conceived the study, analyzed and interpreted the data; PM and RR-V wrote the paper and performed the statistical analyses; BB, DM-C, IC, AL, JS, MJA, FL-C, IN, IL, MS, JP, JM, NC, IJ and GFS reviewed the manuscript and contributed to the final draft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Montesinos, P., Rodríguez-Veiga, R., Boluda, B. et al. Incidence and risk factors of post-engraftment invasive fungal disease in adult allogeneic hematopoietic stem cell transplant recipients receiving oral azoles prophylaxis. Bone Marrow Transplant 50, 1465–1472 (2015). https://doi.org/10.1038/bmt.2015.181
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.181
This article is cited by
-
Prophylactic use amphotericin B use in patients with hematologic disorders complicated by neutropenia: a systematic review and meta-analysis
Scientific Reports (2023)
-
High incidence of resistant breakthrough invasive fungal infections (IFD) in patients treated for acute gastrointestinal graft-versus-host disease (GI GVHD) following allogeneic haematopoietic cell transplantation
Bone Marrow Transplantation (2022)
-
Allogeneic Hematopoietic Stem Cell Transplant in a Pediatric Patient with Invasive Fungal Infections: Challenges and Indications
Current Fungal Infection Reports (2021)
-
Cytomegalovirus reactivation is associated with an increased risk of late-onset invasive aspergillosis independently of grade II–IV acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation: JSTCT Transplant Complications Working Group
Annals of Hematology (2021)
-
Theory-driven development of a medication adherence intervention delivered by eHealth and transplant team in allogeneic stem cell transplantation: the SMILe implementation science project
BMC Health Services Research (2020)