Abstract
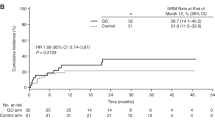
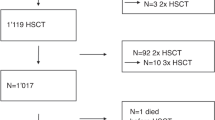
I.v. BU is frequently used in the conditioning regimen prior to allogeneic hematopoietic SCT (allo-HSCT); however, overall outcomes, incidence of hepatic sinusoidal obstructive syndrome (SOS) and its risk factors are not well known. With this aim, we performed a study on 257 AML adult recipients. Seattle Criteria were used for diagnosis and classification of SOS. The median age was 44 years. Donors were HLA-identical siblings in 60%, HLA-matched unrelated in 29% and HLA mismatched in 11%. Conditioning regimen was myeloablative in 84% (i.v. BU with CY was the most frequently used regimen) and it was reduced intensity in 16% (i.v. BU associated with fludarabine). Acute and chronic GVHD was observed in 28% and 44%, respectively. Two-year incidence of non-relapse mortality was 16±2% and 2-year leukemia-free survival for patients in CR1, CR2 and non remission at HSCT were 55±4%, 58±7%, and 20±5%, respectively. At 6 months, incidence of SOS was 7.8±2%; and it was severe in eight patients (3%). Factors associated with the occurrence of SOS were: HLA-mismatched donor HSCT (P=0.002) and patients transplanted in non-remission (P=0.002). In conclusion, outcomes of HSCT using i.v. BU are encouraging in this setting, SOS incidence is low and it is influenced by the type of donor and disease status at the time of transplant.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Thomas E, Storb R, Clift RA, Fefer A, Johnson FL, Neiman PE et al. Bone-marrow transplantation (first of two parts). N Engl J Med 1975; 292: 832–843.
Armitage JO . Bone marrow transplantation. N Engl J Med 1994; 330: 827–838.
Bolinger AM, Zangwill AB, Slattery JT, Glidden D, DeSantes K, Heyn L et al. An evaluation of engraftment, toxicity and busulfan concentration in children receiving bone marrow transplantation for leukemia or genetic disease. Bone Marrow Transplant 2000; 25: 925–930.
Slattery JT, Sanders JE, Buckner CD, Schaffer RL, Lambert KW, Langer FP et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant 1995; 16: 31–42.
Tran HT, Madden T, Petropoulos D, Worth LL, Felix EA, Sprigg-Saenz HA et al. Individualizing high-dose oral busulfan: prospective dose adjustment in a pediatric population undergoing allogeneic stem cell transplantation for advanced hematologic malignancies. Bone Marrow Transplant 2000; 26: 463–470.
Hassan M . The role of busulfan in bone marrow transplantation. Med Oncol 1999; 16: 166–176.
Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant 1996; 17: 225–230.
Grochow LB, Jones RJ, Brundrett RB, Braine HG, Chen TL, Saral R et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1989; 25: 55–61.
Ljungman P, Hassan M, Bekassy AN, Ringden O, Oberg G . High busulfan concentrations are associated with increased transplant-related mortality in allogeneic bone marrow transplant patients. Bone Marrow Transplant 1997; 20: 909–913.
McCune JS, Gooley T, Gibbs JP, Sanders JE, Petersdorf EW, Appelbaum FR et al. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 2002; 30: 167–173.
Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood 1997; 89: 3055–3060.
Bolinger AM, Zangwill AB, Slattery JT, Risler LJ, Glidden DV, Sultan DH et al. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation. Bone Marrow Transplant 2001; 28: 1013–1018.
Deeg HJ, Storer B, Slattery JT, Anasetti C, Doney KC, Hansen JA et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood 2002; 100: 1201–1207.
Andersson BS, Gajewski J, Donato M, Giralt S, Gian V, Wingard J et al. Allogeneic stem cell transplantation (BMT) for AML and MDS following i.v. busulfan and cyclophosphamide (i.v. BuCy). Bone Marrow Transplant 2000; 25: S35–S38.
Andersson BS, Kashyap A, Gian V, Wingard JR, Fernandez H, Cagnoni PJ et al. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant 2002; 8: 145–154.
Andersson BS, Madden T, Tran HT, Hu WW, Blume KG, Chow DS et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant 2000; 6: 548–554.
Schuler US, Renner UD, Kroschinsky F, Johne C, Jenke A, Naumann R et al. Intravenous busulphan for conditioning before autologous or allogeneic human blood stem cell transplantation. Br J Haematol 2001; 114: 944–950.
Shimoni A, Bielorai B, Toren A, Hardan I, Avigdor A, Yeshurun M et al. Intravenous busulfan-based conditioning prior to allogeneic hematopoietic stem cell transplantation: myeloablation with reduced toxicity. Exp Hematol 2003; 31: 428–434.
Carreras E, Díaz-Beyá M, Rosiñol L, Martínez C, Fernández-Avilés F, Rovira M . The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant 2011; 17: 1713–1720.
Thall PF, Champlin RE, Andersson BS . Comparison of 100-day mortality rates associated with i.v. busulfan and cyclophosphamide vs other preparative regimens in allogeneic bone marrow transplantation for chronic myelogenous leukemia: Bayesian sensitivity analyses of confounded treatment and center effects. Bone Marrow Transplant 2004; 33: 1191–1199.
Andersson BS, de Lima M, Thall PF, Wang X, Couriel D, Korbling M et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant 2008; 14: 672–684.
de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 2004; 104: 857–864.
Perkins JB, Kim J, Anasetti C, Fernandez HF, Perez LE, Ayala E et al. Maximally tolerated busulfan systemic exposure in combination with fludarabine as conditioning before allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18: 1099–1107.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
Pai RK, van Besien K, Hart J, Artz AS, O'Donnell PH . Clinicopathologic features of late-onset veno-occlusive disease/sinusoidal obstruction syndrome after high dose intravenous busulfan and hematopoietic cell transplant. Leuk Lymphoma 2012; 53: 1552–1557.
Hasegawa S, Horibe K, Kawabe T, Kato K, Kojima S, Matsuyama T et al. Veno-occlusive disease of the liver after allogeneic bone marrow transplantation in children with hematologic malignancies: incidence, onset time and risk factors. Bone Marrow Transplant 1998; 22: 1191–1197.
Blaise D, Maraninchi D, Archimbaud E, Reiffers J, Devergie A, Jouet JP et al. Allogeneic bone marrow transplantation for acute myeloid leukemia in first remission: a randomized trial of a busulfan-Cytoxan versus Cytoxan-total body irradiation as preparative regimen: a report from the Group d'Etudes de la Greffe de Moelle Osseuse. Blood 1992; 79: 2578–2582.
Ringden O, Ruutu T, Remberger M, Nikoskelainen J, Volin L, Vindelov L et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group. Blood 1994; 83: 2723–2730.
Ferry C, Socie G . Busulfan-cyclophosphamide versus total body irradiation-cyclophosphamide as preparative regimen before allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia: what have we learned? Exp Hematol 2003; 31: 1182–1186.
Morgan M, Dodds A, Atkinson K, Szer J, Downs K, Biggs J . The toxicity of busulphan and cyclophosphamide as the preparative regimen for bone marrow transplantation. Br J Haematol 1991; 77: 529–534.
Richardson PG, Soiffer RJ, Antin JH, Uno H, Jin Z, Kurtzberg J et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol Blood Marrow Transplant 2010; 16: 1005–1017.
Gooley TA, Leisenring W, Cullis JO, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assos 1958; 53: 457–481.
Sobocinski K, Thall P, Bekele B, Klein J, Lennon S, Horwitz M et al. Matched pairs analysis of IV vs PO Busulfan as a conditioning agent prior to transplantion. Blood. 104 Abs 2004; 349.
Johnson DB, Savani BN . How can we reduce hepatic veno-occlusive disease-related deaths after allogeneic stem cell transplantation? Exp Hematol 40: 513–517 2012.
Suyanı E, Altındal Ş, Akı Ş, Sucak GT . Does fludarabine increase the incidence of sinusoidal obstruction syndrome when combined with Bu/Cy during conditioning? Clin Transplant 26: E85–E86 2012.
Rocha V, Porcher R, Fernandes JF, Filion A, Bittencourt H, Silva W Jr et al. Association of drug metabolism gene polymorphisms with toxicities, graft-versus-host disease and survival after HLA-identical sibling hematopoietic stem cell transplantation for patients with leukemia. Leukemia 2009; 23: 545–556.
Ritter CA, Sperker B, Grube M, Dressel D, Kunert-Keil C, Kroemer HK . Overexpression of glutathione S-transferase A1-1 in ECV 304 cells protects against busulfan mediated G2-arrest and induces tissue factor expression. Br J Pharmacol 2002; 137: 1100–1106.
Bredschneider M, Klein K, Mürdter TE, Marx C, Eichelbaum M, Nüssler AK et al. Genetic polymorphisms of glutathione S-transferase A1, the major glutathione S-transferase in human liver: consequences for enzyme expression and busulfan conjugation. Clin Pharmacol Ther 2002; 71: 479–487.
Alatrash G, de Lima M, Hamerschlak N, Pelosini M, Wang X, Xiao L et al. Myeloablative reduced-toxicity i.v. busulfan-fludarabine and allogeneic hematopoietic stem cell transplant for patients with acute myeloid leukemia or myelodysplastic syndrome in the sixth through eighth decades of life. Biol Blood Marrow Transplant 2011; 17: 1490–1496.
Andersson BS, de Lima M, Thall PF, Madden T, Russell JA, Champlin RE . Reduced-toxicity conditioning therapy with allogeneic stem cell transplantation for acute leukemia. Curr Opin Oncol 2009; 21: S11–S15.
Almog S, Kurnik D, Shimoni A, Loebstein R, Hassoun E, Gopher A et al. Linearity and stability of intravenous busulfan pharmacokinetics and the role of glutathione in busulfan elimination. Biol Blood Marrow Transplant 2011; 17: 117–123.
Ferrara JL . Pathogenesis of acute graft-versus-host disease: cytokines and cellular effectors. J Hematother Stem Cell Res 2000; 9: 299–306 Review.
Harris AC, Ferrara JL, Braun TM, Holler E, Teshima T, Levine JE et al. Plasma biomarkers of lower gastrointestinal and liver acute GVHD. Blood 2012; 22: 2960–2963.
Veal GJ, Nguyen L, Paci A, Riggi M, Amiel M, Valteau-Couanet D et al. Busulfan pharmacokinetics following intravenous and oral dosing regimens in children receiving high-dose myeloablative chemotherapy for high-risk neuroblastoma as part of the HR-NBL-1/SIOPEN trial. Eur J Cancer 2012; 48: 3063–3072.
Corbacioglu S, Cesaro S, Faraci M, Valteau-Couanet D, Gruhn B, Rovelli A et al. ValtDefibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet 2012; 379: 1301–1309.
Acknowledgements
This study was supported by an unrestricted educational grant from Pierre-Fabre Medicament. Vanderson Rocha was supported, in part, by the National Institute for Health Research (NIHR), Oxford Biomedical Research Center at Oxford University Hospitals NHS Trust and University of Oxford. We thank all European Blood and Marrow Transplant centers and national registries for contributing patients to the study (Supplement) and Data Managers for their superb work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M. Mohty, A. Nagler and V. Rocha received lecture honoraria from Pierre-Fabre Medicament whose product is discussed in this paper.
Rights and permissions
About this article
Cite this article
Nagler, A., Labopin, M., Berger, R. et al. Allogeneic hematopoietic SCT for adults AML using i.v. BU in the conditioning regimen: outcomes and risk factors for the occurrence of hepatic sinusoidal obstructive syndrome. Bone Marrow Transplant 49, 628–633 (2014). https://doi.org/10.1038/bmt.2014.7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.7
Keywords
This article is cited by
-
Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European society for blood and marrow transplantation
Bone Marrow Transplantation (2018)
-
Risk of sinusoidal obstruction syndrome in allogeneic stem cell transplantation after prior gemtuzumab ozogamicin treatment: a retrospective study from the Acute Leukemia Working Party of the EBMT
Bone Marrow Transplantation (2017)
-
Safety and efficacy of thiotepa-based conditioning for allogeneic transplantation in AML: a survey from the ALWP of the EBMT
Bone Marrow Transplantation (2017)
-
Toxicity and efficacy of busulfan and fludarabine myeloablative conditioning for HLA-identical sibling allogeneic hematopoietic cell transplantation in AML and MDS
Bone Marrow Transplantation (2016)
-
Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation
Bone Marrow Transplantation (2016)