Abstract
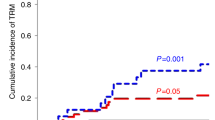
Availability of an HLA-identical sibling (MRD) or suitably matched unrelated donor (MUD) has historically been a limiting factor in the application of allogeneic hematopoietic transplantation. Although almost all patients have an HLA-haploidentical family donor, prior attempts at transplantation from such donors using T-cell replete grafts and conventional immunosuppression were associated with unacceptable rates of GVHD, and when stringent ex vivo T-cell depletion was used to control GVHD, rates of graft rejection and post-transplant infections were prohibitive. The recent approach to HLA-haploidentical donor transplantation developed in Baltimore that uses T-cell replete grafts and post-transplant CY (Haplo-post-HCT-CY) to control post-transplant allo-reactivity appears to have overcome many of the obstacles historically associated with haploidentical donor transplantation. In particular, TRM rates of <10% are usual and rapid reconstitution of immunity leads to a low rate of post-transplant infections and no post-tranplant lymphoproliferative disorders (PTLD), consistent with the hypothesis that post-transplant CY selectively depletes proliferating alloreactive T cells responsible for GVHD and graft rejection while preserving resting memory T cells essential for post-transplant immunologic recovery. In parallel trials using similar non-myeloablative conditioning regimens, Haplo-post-HCT-CY produced similar overall survival to double umbilical cord blood transplantation(DUCBT) in adult patients (62% vs 54%), with low rates of TRM (7% vs 24%), severe acute GVHD (0% vs 21%) and chronic GVHD (13% vs 25%). Furthermore, recent non-randomized comparisons adjusted for risk factors show that Haplo-post-HCT-CY achieve at least equivalent outcomes to conventional MRD and MUD transplants. Although most experience has been obtained using BM, emerging data suggest that a G-CSF mobilized PBSC graft can also safely be used for Haplo-post-HCT-CY. Haplo-post-HCT-CY also avoids the graft acquisition costs of DUCBT and MUDs and the cost of cell selection associated with T-depleted grafts. Although randomized comparisons will be forthcoming, Haplo-post-HCT-CY can already be considered a valid standard-of-care in patients who lack conventional donors thus extending the availability of allogeneic transplants to almost all patients. This donor source may also challenge the routine preference for a MUD in patients lacking an MRD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Anasetti C, Beatty PG, Storb R, Martin PJ, Mori M, Sanders JE et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol 1990; 29: 79–91.
Beatty PG, Clift RA, Mickelson EM, Nisperos BB, Flournoy N, Martin PJ et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med 1985; 313: 765–771.
Powles RL, Morgenstern GR, Kay HE, McElwain TJ, Clink HM, Dady PJ et al. Mismatched family donors for bone-marrow transplantation as treatment for acute leukaemia. Lancet 1983; 1: 612–615.
Szydlo R, Goldman JM, Klein JP, Gale RP, Ash RC, Bach FH et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol 1997; 15: 1767–1777.
Ash RC, Horowitz MM, Gale RP, van Bekkum DW, Casper JT, Gordon-Smith EC et al. Bone marrow transplantation from related donors other than HLA-identical siblings: effect of T cell depletion. Bone Marrow Transplant 1991; 7: 443–452.
O'Reilly RJ, Keever C, Kernan NA, Brochstein J, Collins N, Flomenberg N et al. HLA nonidentical T cell depleted marrow transplants: a comparison of results in patients treated for leukemia and severe combined immunodeficiency disease. Transplant Proc 1987; 19: 55–60.
Mehta J, Singhal S, Gee AP, Chiang KY, Godder K, Rhee Fv F et al. Bone marrow transplantation from partially HLA-mismatched family donors for acute leukemia: single-center experience of 201 patients. Bone Marrow Transplant 2004; 33: 389–396.
Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol 2005; 23: 3447–3454.
Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 2011; 117: 3921–3928.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W et al. Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant 2009; 15: 257–265.
Rizzieri DA, Koh LP, Long GD, Gasparetto C, Sullivan KM, Horwitz M et al. Partially matched, nonmyeloablative allogeneic transplantation: clinical outcomes and immune reconstitution. J Clin Oncol 2007; 25: 690–697.
Berenbaum MC, Brown IN . Prolongation of Homograft Survival in Mice with Single Doses of Cyclophosphamide. Nature 1963; 200: 84.
Nomoto K, Eto M, Yanaga K, Nishimura Y, Maeda T . Interference with cyclophosphamide-induced skin allograft tolerance by cyclosporin A. J Immunol 1992; 149: 2668–2674.
Kastan MB, Schlaffer E, Russo JE, Colvin OM, Civin CI, Hilton J . Direct demonstration of elevated aldehyde dehydrogenase in human hematopoietic progenitor cells. Blood 1990; 75: 1947–1950.
Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ . Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood 2001; 98: 3456–3464.
Ross D, Jones M, Komanduri K, Levy RB . Antigen and lymphopenia-driven donor T cells are differentially diminished by post-transplantation administration of cyclophosphamide after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2013; 19: 1430–1438.
Kanakry CG, Ganguly S, Zahurak M, Bolanos-Meade J, Thoburn C, Perkins B et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med 2013; 5: 211ra157.
O'Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2002; 8: 377–386.
Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008; 14: 641–650.
Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011; 118: 282–288.
Kasamon YL, Luznik L, Leffell MS, Kowalski J, Tsai HL, Bolanos-Meade J et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant 2010; 16: 482–489.
Grosso D, Carabasi M, Filicko-O'Hara J, Kasner M, Wagner JL, Colombe B et al. A 2-step approach to myeloablative haploidentical stem cell transplantation: a phase 1/2 trial performed with optimized T-cell dosing. Blood 2011; 118: 4732–4739.
Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK et al. Haploidentical Transplantation Using T Cell Replete Peripheral Blood Stem Cells and Myeloablative Conditioning in Patients with High-Risk Hematologic Malignancies Who Lack Conventional Donors is Well Tolerated and Produces Excellent Relapse-Free Survival: Results of a Prospective Phase II Trial. Biol Blood Marrow Transplant 2012; 18: 1859–1866.
Jacobson S, OCN, Sanacore M, Zhang X, Sizemore C, Brown S et al. Myeloablative Conditioning With PBSC Grafts For T-Replete Haploidentical Donor Hematopoietic Cell Transplantation Using Post-Transplant Cyclophosphamide Results In Universal Engraftment, Low Rates Of Gvhd, NRM and Excellent Survival Outcomes: An Analysis Of Two Consecutive Phase II Studies From a Single Center. Blood 2013; 122: 3351.
Symons HJ, Chen A, Luznik L, Kasamon YL, Bolanos-Meade, Jones RJ et al. Myeloablative Haploidentical Bone Marrow Transplantation with T Cell Replete Grafts and Post-Transplant Cyclophosphamide: Results of a Phase II Clinical Trial. Am Soc Hematol Proc, Blood 2011, abstract 4151.
Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant 2013; 19: 117–122.
Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2012; 18: 1835–1844.
Jacobson CA, Turki AT, McDonough SM, Stevenson KE, Kim HT, Kao G et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2012; 18: 565–574.
Ruggeri A, Peffault de Latour R, Carmagnat M, Clave E, Douay C, Larghero J et al. Outcomes, infections, and immune reconstitution after double cord blood transplantation in patients with high-risk hematological diseases. Transpl Infect Dis 2011; 13: 456–465.
Curtis RE, Travis LB, Rowlings PA, Socie G, Kingma DW, Banks PM et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood 1999; 94: 2208–2216.
Ballen KK, Cutler C, Yeap BY, McAfee SL, Dey BR, Attar EC et al. Donor-derived second hematologic malignancies after cord blood transplantation. Biol Blood Marrow Transplant 2010; 16: 1025–1031.
Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik JA et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood 2006; 108: 2874–2880.
Dominietto A, Raiola AM, Bruno B, van Lint MT, Frassoni F, Di Grazia C et al. Rapid Immune Reconstitution Following Unmanipulated Haploidentical BMT with Post-Transplant High Dose Cyclophosphamide. Blood 2011; 118: 1315–1315.
Kanakry JA, Kasamon YL, Bolanos-Meade J, Borrello IM, Brodsky RA, Fuchs EJ et al. Absence of Post-Transplantation Lymphoproliferative Disorder after Allogeneic Blood or Marrow Transplantation Using Post-Transplantation Cyclophosphamide as Graft-versus-Host Disease Prophylaxis. Biol Blood Marrow Transplant 2013; 19: 1514–1517.
Komanduri KV St, John LS, de Lima M, McMannis J, Rosinski S, McNiece I et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood 2007; 110: 4543–4551.
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol 2013; 31: 1310–1316.
Burroughs LM, O'Donnell PV, Sandmaier BM, Storer BE, Luznik L, Symons HJ et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 2008; 14: 1279–1287.
Castagna L, Bramanti S, Furst S, Sarina B, El Cheikh J, Granata A et al. Lower Relapse and Better PFS Among Chemosensitive Patients Undergoing Allogeneic Transplantation By Haploidentical Compared With HLA-Identical DONOR: Results On A Cohort Of 94 Patients With Hodgkin’S Lymphoma. Blood 2013; 122: 2144.
Raiola A, Dominietto A, Varaldo R, Ghiso A, Galaverna F, Bramanti S et al. Unmanipulated haploidentical BMT following non-myeloablative conditioning and post-transplantation CY for advanced Hodgkin's lymphoma. Bone Marrow Transplant 2013; 49: 190–194.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W . Donor lymphocyte infusion for the treatment of leukemia relapse after HLA-mismatched/haploidentical T-cell-replete hematopoietic stem cell transplantation. Haematologica 2007; 92: 414–417.
Forde P, Symons HJ, Chen AR, Smith BD, Fuchs EJ, Luznik L et al. The Use Of Donor Lymphocyte Infusion (DLI) For Relapse After Related T-Cell Replete HLA-Haploidentical Bone Marrow Transplantation (haploBMT) With Posttransplantation Cyclophosphamide (PTCy). Blood 2013; 122: 4629.
Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood 2008; 112: 3574–3581.
Federmann B, Bornhauser M, Meisner C, Kordelas L, Beelen DW, Stuhler G et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: a phase II study. Haematologica 2012; 97: 1523–1531.
Gladstone DE, Zachary AA, Fuchs EJ, Luznik L, Kasamon YL, King KE et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant 2013; 19: 647–652.
Symons HJ, Leffell MS, Rossiter ND, Zahurak M, Jones RJ, Fuchs EJ . Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant 2010; 16: 533–542.
Huang XJ . Donor Selection For Haploidentical Hematopoietic Stem Cell Transplantation: Who Is The Better – Donor-Recipient Risk Factor Analysis. Blood 2013; 122: 705.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bashey, A., Solomon, S. T-cell replete haploidentical donor transplantation using post-transplant CY: an emerging standard-of-care option for patients who lack an HLA-identical sibling donor. Bone Marrow Transplant 49, 999–1008 (2014). https://doi.org/10.1038/bmt.2014.62
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.62
This article is cited by
-
Severe infections and infection-related mortality in a large series of haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide
Bone Marrow Transplantation (2021)
-
Single-Center Study of 72 Patients with Severe Combined Immunodeficiency: Clinical and Laboratory Features and Outcomes
Journal of Clinical Immunology (2021)
-
Uniform graft-versus-host disease prophylaxis with posttransplant cyclophosphamide, sirolimus, and mycophenolate mofetil following hematopoietic stem cell transplantation from haploidentical, matched sibling and unrelated donors
Bone Marrow Transplantation (2020)
-
Hematopoietic Stem Cell Transplantation in Pediatric Acute Lymphoblastic Leukemia
Current Hematologic Malignancy Reports (2019)
-
Allogeneic Hematopoietic Stem Cell Transplantation for Myeloma: Time for an Obituary or Not Just Yet!
Indian Journal of Hematology and Blood Transfusion (2019)