Abstract
In all, 661 of 680 centers in 48 countries reported 37 818 hematopoietic SCT (HSCT) in 33 678 patients (14 165 allogeneic (42%), 19 513 autologous (58%)) in the 2012 survey. Main indications were leukemias, 10 641 (32%; 95% allogeneic); lymphoid neoplasias, 19 336 (57%; 11% allogeneic); solid tumors, 1630 (5%; 3% allogeneic); and nonmalignant disorders, 1953 (6%; 90% allogeneic). There were more unrelated donors than HLA-identical sibling donors (54% versus 38% (8% being mismatched related donor HSCT)). Cord blood was almost exclusive in allogeneic transplants (5% of total). Since 2011, the highest increases in allogeneic HSCT were for AML in CR1 (12%) and for myeloproliferative neoplasm (15%). For autologous HSCT the main increases were for plasma cell disorders (7%), non-Hodgkin lymphoma (4%) and autoimmune disease (50%). There were 4097 pediatric patients <18 years of age receiving HSCT, 2902 received an allogeneic and 1195 an autologous HSCT. Overall, 69% of allogeneic and 64% of autologous HSCT were performed in dedicated pediatric centers and the remainder in combined adult and pediatric centers. Distributions of diseases, donor types and stem cell source for all patients and pediatric patients in particular are shown. A percentage of centers fulfilling the annual required criteria for patient numbers for JACIE accreditation are provided.
Similar content being viewed by others
Introduction
Hematopoietic SCT (HSCT) is an established procedure for many acquired and congenital disorders of the hematopoietic system, including disorders of the immune system, and as enzyme replacement in metabolic disorders.1, 2, 3, 4 The annual activity survey of the European Society of Blood and Marrow Transplantation (EBMT), describing the status of HSCT in Europe and affiliated countries, has become an instrument that is used to observe trends and to monitor changes in technology use.5, 6, 7, 8, 9, 10 The survey captures the numbers of HSCT performed in the preceding year from each participating team, divided by indication, donor type and stem cell source. The standardized structure of the survey over many years and the excellent commitment of the participating teams allow us to observe changes over time and to evaluate factors associated with these changes. More recently, the survey has included additional information on novel cell therapies with hematopoietic stem cells for non-hematopoietic use, as well as on the use of non-hematopoietic stem and progenitor cells.11 This coincides with the recent interest of the World Health Organization (WHO; www.who.org) in cell and tissue transplants and further stresses the need for adequate and timely information (http://www.who.int/topics/transplantation/en/). The analysis of the survey data spanning over 20 years has shown a continued and constant increase in the annual numbers of HSCT and transplant rates (number of HSCT/10 million inhabitants) for both allogeneic and autologous HSCT.
This report is based on the 2012 survey data. In addition to transplant rates and indications, stem cell source and donor type in allogeneic HSCT, the activity for adult and pediatric HSCT is collected separately.
Patients and methods
Data collection and validation
Participating teams were invited to report data for 2012 by indication, stem cell source and donor type as listed in Table 1. The survey allows the reporting of additional information on the numbers of subsequent transplants performed as a result of relapse, rejection or those that are part of a planned sequential transplant protocol. Supplementary information on the numbers of DLI, reduced-intensity HSCT and the numbers of pediatric HSCT is also collected. Quality control measures included several independent systems: confirmation of validity of the entered data by the reporting team, selective comparison of the survey data with MED-A data sets in the EBMT Registry database and cross-checking with the National Registries.
Teams
In all, 680 centers from 48 countries were contacted for the 2012 survey (38 European and 10 affiliated countries), of which 661 teams from 48 countries (38 European, 10 affiliated countries) reported their numbers. This corresponds to a 97% return rate and includes 543 active EBMT member teams. An additional 19 active teams failed to report in 2012 and 6 teams reported no activity due to transplant program development or closure.
Contacted teams are listed in the online appendix (Supplementary Information) in alphabetical order by country, city, EBMT center code, with their reported numbers of first and total HSCT, and of first allogeneic and autologous HSCTs. The WHO regional office definitions (www.who.org) were used to classify countries as European or non-European. According to information received, there were no blood or marrow transplants performed in Albania, Andorra, Armenia, Georgia, Liechtenstein, Malta, Moldavia, Monaco, Montenegro and San Marino in 2012. Ten non-European countries participated in the 2012 EBMT survey: Algeria, Kazakhstan, Iran, Israel, Jordan, Lebanon, Nigeria, Saudi Arabia, South Africa and Tunisia. Their data, 6.6% of the total data set, are included in all analyses.
Definitions
Patient and transplant numbers
Wherever appropriate, patient numbers corresponding to the number of patients receiving a first transplant and transplant numbers reflecting the total number of transplants performed are listed. Multiple transplants may include multiple transplants defined as subsequent transplants within a planned double or triple autologous or allogeneic transplant protocol, and retransplants (autologous or allogeneic) defined as unplanned HSCT for rejection or relapse after a previous HSCT. New in 2012 is the overview of pediatric HSCT defined as transplants for patients <18 years of age. All centers performing HSCT on patients above and below the age of 18 were considered combined adult and pediatric centers. There were 56 (25 allogeneic and 31 autologous) patients reported by 21 dedicated pediatric centers who were >18 years of age at time of transplant. These centers continued to be counted as dedicated pediatric centers.
As a result of these data, we are now able to calculate the percentages of centers fulfilling the annual number of transplant requirements for JACIE accreditation as specified by the Foundation for the Accreditation of Cell Therapy (FACT)–JACIE standard using the 2012 activity survey data (http://www.jacie.org/). Although there is no definition of age for pediatric patients in FACT–JACIE standards, the EBMT pediatric disease working party (PDWP) defines pediatric patients according to the International Conference of Harmonization as individuals up to 18 years of age. Information on stem cell source includes BM, peripheral blood or cord blood; transplants with more than one source were categorized as cord blood HSCT if cord blood was present or peripheral blood HSCT if BM and peripheral blood were used.
Data collection included information on the use of reduced-intensity conditioning transplants, as defined by EBMT
Information on additional cellular therapies was subdivided into: HSC for non-hematopoietic use; non-hematopoietic stem cell therapies; MSC therapies for rejection or GVHD prevention/treatment; and DLI. Collection of information was validated by cross-checking with a similar more detailed survey carried out by TERMIS-EU (Tissue Engineering and Regenerative Medicine International Society; www.termis.org), EULAR (European League against Rheumatism; www.eular.org), ICRS-EU (International Cartilage Repair Society; www.cartilage.org) and ISCT (International Society of Cellular Therapy; www.celltherapysociety.org).11
Transplant rates
Transplant rates, defined as numbers of HSCT per 10 million inhabitants, were computed for each country without adjustments for patients who crossed borders and received their HSCT in a foreign country. Population numbers were obtained from Eurostats for the European countries (http://epp.eurostat.ec.europa.eu/portal/page/portal/statistics/search_database) and the US census bureau database for the non-European countries (http://www.census.gov/population/international/data/idb/rank.php).
Analysis
Wherever appropriate absolute numbers of transplanted patients, transplants or transplant rates are shown for specific countries, indications or transplant techniques.
Results
2012 Data
Participating teams in 2012
Of the 661 teams, 411 (62%) performed both allogeneic and autologous transplants; 229 (35%) restricted their activity to autologous HSCT only; 15 teams (2%) to allogeneic transplants only; and 6 teams (1%) reported having performed no transplants in 2012 because of renovation or temporary closure of the transplant unit.
Overall, 118 (18%) centers performed transplants on both adult and pediatric patients; 109 (16%) centers were dedicated pediatric transplant centers; and 434 (66%) centers performed transplants on adults only (29 581 transplants; 11 263 allogeneic and 18 318 autologous).
Numbers of patients and transplants
A total of 33 378 patients received their first transplant in 2012. Of these, 14 165 (42%) were allogeneic and 19 513 (58%) autologous.
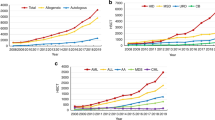
Furthermore, there were 2501 retransplants (1098 allogeneic/1403 autologous) and 1639 multiple transplants (88 allogeneic/1551 autologous), bringing the total to 37 818 HSCT procedures, 15 351 allogeneic (41%) and 22 467 autologous (59%) procedures performed in 2012, which is an increase of 6% compared with 2011 and a 30% and 56% increase compared with 2007 and 2002, respectively.12
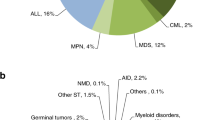
Indications for HSCT in 2012 are listed in detail in Table 1. Main indications were leukemias, 10 641 (32% of total; 95% of which were allogeneic); lymphoid neoplasias including non-Hodgkin's lymphoma, Hodgkin lymphoma and plasma cell disorders, 19 336 (57%; 11% allogeneic); solid tumors, 1630 (5%; 3% allogeneic); and nonmalignant disorders, 1953 (6%; 90% allogeneic). As seen in previous years, the majority of HSCT for lymphoid malignancies were autologous, whereas most of the transplants for leukemia were performed using stem cells from allogeneic donors. Autologous HSCT for nonmalignant disorders predominantly includes patients with autoimmune disorders.
Figures 1a and b show transplant rates by country for allogeneic and autologous HSCT. Median transplant rates per 10 million inhabitants were 112 (range, 0.1–561) for allogeneic HSCT and 235 (range, 3.4–667) for autologous HSCT.
Distributions of indications for HSCT are shown in Figures 2a and b for allogeneic and autologous HSCT, respectively.
When compared with 2011 the total number of transplants increased by 6% (5.5% allogeneic HSCT and 6.4% autologous HSCT).13 The number of unrelated donor transplants increased by 5.4% from 7799 to 8224. The most significant increases in allogeneic HSCT were for AML in CR1 (12%) and for myeloproliferative neoplasm (15%). For autologous HSCT there was a decrease in activity for acute leukemia (8% for AML and 22% for ALL) but an increase for plasma cell disorders by 7% and non-Hodgkin's lymphoma by 4%. Autologous HSCT for autoimmune disease had increased by 50%.
Stem cell source and donor type
There were clear differences in the use of stem cell sources between autologous and allogeneic HSCT. Of the 22 467 autologous transplants, 176 (1%) were BM derived and 22 285 (99%) were derived from PBSC or from combined peripheral blood and BM. Only six autologous cord blood HSCT were reported for 2012.
Of the 15 351 allogeneic transplants, 3476 (23%) were BM, 11 117 (72%) were peripheral blood and 758 (5%) were cord blood transplants. BM remained the preferred source of stem cells for allogeneic transplants for nonmalignant disorders (62%). Cord blood was used as stem cell source from unrelated donors in 694 (91%), from HLA-identical siblings in 58 (8%), 6 (0.1%) were from other family members and 6 were autologous. Overall, the use of cord blood as a stem cell source was slightly reduced from 833 in 2011 to 758 in 2012. The highest incidence of cord blood transplants from unrelated donors was seen in France, Spain, Italy, the UK and the Netherlands: 511 (74% of all unrelated cord blood HSCT reported to the EBMT). On the contrary, use of alternative related donors, mainly haploidentical donors increased by 24% to 1217.
Donors for the 15 351 allogeneic HSCT were HLA-identical siblings (5806; 38%), other family members (1217; 8%), syngeneic twin donors (46; 0.3%), unrelated BM or peripheral blood donors (7530; 49%) or unrelated cord blood donors (694; 4%). The percentage of unrelated donor HSCT continues to increase and has reached 54% of all allogeneic HSCT in 2012.14
Reduced-intensity conditioning
Numbers of reduced-intensity conditioning HSCT continued to increase from 1436 in 2000 to 5865 in 2012.15 Reduced-intensity conditioning was used for 38% of all allogeneic HSCT, a proportion similar to that of the previous year’s survey.
DLI
In 1997, 305 patients were reported as having received DLI after transplant; this has increased to 2251 in 2012 and corresponds to 15% of patients with an allogeneic HSCT.
Additional cellular therapies
Twenty-one teams from 11 countries reported having treated 189 patients with hematopoietic stem cells for non-hematopoietic use in 2012. The main indications were cardiovascular, 152 (140 autologous); neurological, 20 (all autologous); tissue repair, 9 (7 autologous) and epithelial, 8 (5 autologous). In addition, 403 patients in 75 teams and 18 countries received mesenchymal stromal cellsfor prevention/treatment of GVHD (344), prevention/treatment of graft failure (21) and for unspecified reasons (38).
Numbers of pediatric patients and transplants
New in the 2012 survey is the possibility of reporting the number of pediatric transplants performed either in dedicated pediatric transplant centers or in centers performing transplants in both adult (>18 years of age at transplant) and pediatric patients.
In all, 4041 transplants, 2877 (71%) allogeneic and 1164 (29%) autologous, were reported in patients under the age of 18 years at transplant. An additional 56 (25 allogeneic and 31 autologous) patients >18 years at the time of transplant were reported by 21 of the dedicated pediatric centers giving a total of 4097 pediatric transplants (2902 allogeneic and 1195 autologous) in 2012.16 The proportion of autologous to allogeneic HSCT is different in pediatrics (29% autologous) compared with adults (62% autologous), and is mainly used for treating solid tumors. The pediatric population has continued to represent ~20% of the entire population and is somewhat out of proportion to the number of adult patients in this population. Also this population, although a minority, is still a significant minority that includes a number of unique diagnoses such as the hemoglobinopathies, immune deficiencies, immune dysregulation and metabolic diseases, all of which are not common or present at all in the adult HSCT population.
Donor type was HLA-identical sibling and twin, 1170 (40%); other family member, 350 (12%); and unrelated, 1382 (48%). Stem cell sources for allogeneic transplants were BM, 1729 (60%); PBSC, 847 (29%); and cord blood, 326 (11%; 51 targeted sibling, 3 other family member and 272 unrelated).
Pediatric transplant rates by country for allogeneic and autologous HSCT are shown in Figures 3a and b. Figure 3c shows pediatric cord blood transplant rates. Transplant rates for allogeneic HSCT (>55 per 10 million population) were reported in Israel, Italy, Saudi Arabia and Slovenia. For autologous HSCT transplant rates (>45 per 10 million population) were reported in Israel and Finland.
Pediatric transplant rates in Europe (= total number of HSCT per 10 million inhabitants) by participating country in 2012 in both center types. (a) Allogeneic transplant rates/10 million population. (b) Autologous transplant rates/10 million population. (c) Cord blood HSCT transplant rates/10 million population.
2760 (67%; 1999 allogeneic and 761 autologous) of the 4097 pediatric patients were performed in dedicated pediatric transplant centers. The remaining 1337 (903 allogeneic and 434 autologous) were performed in centers that performed transplants in both adults and pediatric patients. Figures 4a and b show the percentages of pediatric HSCT performed in dedicated pediatric centers as opposed to combined centers by country for allogeneic and autologous HSCT, respectively.
Absolute numbers and relative proportions of indications for pediatric HSCT in the 109 dedicated centers in Europe in 2012. (a) Proportions of pediatric disease indications for an allogeneic HSCT in Europe in 2012. (b) Proportions of pediatric disease indications for an autologous HSCT in Europe in 2012.
Stem cell source does not differ significantly when comparing pediatric patients transplanted in dedicated pediatric centers (BM 63%, PB 26%, CB 11%) with pediatric patients transplanted in combined adult and pediatric centers (BM 52%, PB 37% CB 11%). Donor types are also comparable (HLA-identical sibling 39% vs 44%; unrelated donors 49% vs 45%; other related donors 12% vs 11%).
Indications for pediatric HSCT are shown in Figures 5a and b and are limited to data from the 109 dedicated pediatric centers. The main indication for allogeneic HSCT is ALL (520; 26%), and primary immune deficiencies (315; 16%). For autologous HSCT, however, it is solid tumors (504; 66% including 267 (53%) neuroblastomas) and lymphomas (111; 15%).
The main indication for 354 HSCT performed using haploidentical family donors (12% of all pediatric allogeneic transplants) was leukemia.
JACIE accreditation criteria
Using the information available from allogeneic and autologous adult and pediatric centers as well as that reported by combined centers, we looked at transplant number requirements for JACIE accreditation and calculated the percentage of centers fulfilling those requirement criteria on the basis of the reported 2012 data. Of centers performing allogeneic (and autologous) adult HSCT, 177 of 219 (81%) fulfilled the criterion of 10 allogeneic HSCT in 2012. Of 209 centers performing only autologous adult HSCT, 191 (91%) fulfilled the criterion of five autologous HSCT in 2012. Of 95 dedicated pediatric-only allogeneic centers, 66 (69%) fulfilled the criterion of 10 allogeneic pediatric HSCT per annum. Of 14 dedicated pediatric autologous only HSCT centers, 6 (43%) fulfilled the criterion of five pediatric autologous HSCT. Of 118 centers that reported both adult and pediatric HSCT activity, 85 were allogeneic (and autologous, as accreditation for allogeneic permits autologous HSCT as well) adult and pediatric combined centers and 46 (54%) fulfilled the criterion of at least five adult and five pediatric allogeneic HSCT in 2012. There were 33 remaining centers performing adult and pediatric autologous HSCT. Of these, 27 performed adult allogeneic HSCT as well and 22 of the 27 (81%) fulfilled the criterion of 10 allogeneic HSCT. Six centers performed only autologous HSCT for adults and children. Of these six centers, all fulfilled the criterion for adult autologous HSCT. For this specific combination (adult and pediatric autologous HSCT) JACIE has not defined a requirement. We did not include the six centers reporting no transplants in 2012 in this calculation.
Discussion
The EBMT activity survey has been conducted annually since 1990. The 2010 survey reported for the first time >30 000 patients transplanted in a given year. This trend continues with an additional increase by 6% in 2012, suggesting that HSCT remains an increasingly important treatment modality in the era of targeted antibody and molecular therapy. The present 2012 report is on 37 818 transplants.
HSCT for some indications continues to increase but not for others. Of interest is the growth of allogeneic HSCT for AML CR1, myeloproliferative neoplasm, plasma cell disorders and primary immunodeficiency disorders. For autologous HSCT, autoimmune diseases saw a significant increase, smaller increases were observed for myeloma and non-Hodgkin's lymphoma, whereas AML CR1 decreased by 11%.
We present data on pediatric transplants performed in dedicated pediatric centers compared with pediatric patients transplanted in combined adult and pediatric centers. Approximately twice as many pediatric patients are transplanted in dedicated pediatric centers when compared with combined pediatric and adult centers. It would be of interest to compare outcomes between these groups but these data are not available. It is also of interest, that more pediatric patients receive BM than PBSC, irrespective of donor type. This is explained by the higher incidence of nonmalignant conditions and the higher risk for chronic GVHD with peripheral blood as a stem cell source. The percentage of patients transplanted in dedicated pediatric centers varies considerably by country, probably related to policies in different countries; it does not appear to be related to the size of the country.
Lastly, we used the data of the 2012 survey to analyze the proportion of centers fulfilling quantitative accreditation criteria of JACIE. A large majority of adult centers do fulfill these criteria; however, for reasons explained by the lower incidence of pediatric indications for HSCT, the percentage of centers fulfilling accreditation criteria is lower for dedicated pediatric centers but even lower for centers with combined adult and pediatric HSCT. These data may serve to advance the discussion of strengthening pediatric competence as opposed to combining forces between adult and pediatric specialists and that of the benefit of proximity for patients as opposed to concentrating competence into fewer but larger and therefore more geographically distant centers.17,18 Readers should keep in mind that accreditation criteria are not based on the activity of a single year and therefore the data presented here should be interpreted acknowledging this fact.
References
Copelan EA . Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354: 1813–1826.
Appelbaum FR . Hematopoietic-cell transplantation at 50. N Engl J Med 2007; 357: 1472–1475.
Ljungman P, Bregni M, Brune M, Cornelissen J, deWitte T, Dini G et al. European Group for Blood and Marrow. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant 2010; 45: 219–234.
Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010; 303: 1617–1624.
Gratwohl A, Baldomero H, Schwendener A, Gratwohl M, Apperley J, Frauendorfer K et al. The EBMT activity survey 2008 impact of team size, team density and new trends. Bone Marrow Transplant 2011; 46: 174–191.
Gratwohl A . Bone marrow transplantation activity in Europe 1990. Report from the European Group for Bone Marrow Transplantation (EBMT). Bone Marrow Transplant 1991; 8: 197–201.
Gratwohl A, Baldomero H, Horisberger B, Schmid C, Passweg J, Urbano-Ispizua A . Accreditation Committee of the European Group for Blood and Marrow Transplantation (EBMT). Current trends in haematopoietic stem cell transplantation in Europe. Blood 2002; 100: 2374–2386.
Gratwohl A, Baldomero H, Schwendener A, Gratwohl M, Apperley J, Niederwieser D et al. Joint Accreditation Committee of the International Society for Cellular Therapy; European Group for Blood and Marrow Transplantation; European Leukemia Net. Predictability of hematopoietic stem cell transplantation rates. Haematologica 2007; 92: 1679–1686.
Gratwohl A, Baldomero H, Schwendener A, Rocha V, Apperley J, Frauendorfer K et al. The EBMT activity survey 2007 with focus on allogeneic HSCT for AML and novel cellular therapies. Bone Marrow Transplant 2009; 43: 275–291.
Gratwohl A, Schwendener A, Baldomero H, Gratwohl M, Apperley J, Niederwieser D et al. Changes in use of hematopoietic stem cell transplantation; a model for diffusion of medical technology. Haematologica 2010; 95: 637–643.
Martin I, Baldomero H, Bocelli-Tyndall C, Emmert MY, Hoerstrup SP, Ireland H et al. The survey on cellular and engineered tissue therapies in Europe in 2011. Tissue Eng Part A 2013; 20: 842–853.
Passweg JR, Baldomero H, Gratwohl A, Bregni M, Cesaro S, Dreger P et al. The EBMT activity survey: 1990-2010. Bone Marrow Transplant 2012; 47: 906–923.
Passweg JR, Baldomero H, Bregni M, Cesaro S, Dreger P, Duarte RF et al. Hematopoietic SCT in Europe: data and trends in 2011. Bone Marrow Transplant 2013; 48: 1161–1167.
Foeken LM, Green A, Hurley CK, Marry E, Wiegand T, Oudshoorn M . Monitoring the international use of unrelated donors for transplantation: the WMDA annual reports. Bone Marrow Transplant 2010; 45: 811–818.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009; 15: 1628–1633.
Schultz RK, Baker KS, Boelens JJ, Bollard CM, Egeler RM, Cowan M et al. Challenges and opportunities for international cooperative studies in pediatric hematopoeitic cell transplantation: priorities of the Westhafen Intercontinental Group. Biol Blood Marrow Transplant 2013; 19: 1279–1287.
Gratwohl A, Brand R, Niederwieser D, Baldomero H, Chabannon C, Cornelissen J et al. Introduction of a Quality Management System and Outcome After Hematopoietic Stem-Cell Transplantation. J Clin Oncol 2011; 29: 1980–1986.
Abou-Nassar E, Kim T, Blossom J, Ho V, Soiffer R, Cutler C et al. The Impact of Geographic Proximity to Transplant Center on Outcomes after Allogeneic Hematopoietic Stem Cell. Biology of Blood and Marrow Transplantation 2012; 18: 708–715.
Acknowledgements
The cooperation of all participating teams and their staff (listed in the Appendix), the EBMT Co-ordination office; Barcelona, Paris, London (C Ruiz de Elvira, S Hewerdine), the Austrian Registry (ASCTR) (H Greinix, B Lindner, C Wagner), the Belgium Registry (Y Beguin, M Van Spauwen) the Czech BMT Registry (P Zak, M Trnkova), the French Registry (SFGM) (N Milpied, N Raus), the German Registry (DRST) (H Ottinger, K Fuchs, C Müller, H Neidlinger, F Strehle), the Italian Registry (GITMO) (A Rambaldi, B Bruno, A Camboni), the Dutch Registry (JJ Cornelissen, M Groenendijk), Spanish BMT Registry (GETH) (J Diez Martin, A Cedillo), the Swiss Registry (SBST) (U Schanz, H Baldomero), the Turkish BMT Registry (G Gurman, M Arat, F Arpaci) and the British Registry (BSBMT) (G Cook, K Kirkland, J Perry) is greatly appreciated. We also thank D John for database support. EBMT is supported by grants from the corporate sponsors: GentiumSpA, Gilead Sciences Europe Ltd, Celgene International SARL, AstellasPharma Europe Ltd, Sanofi Oncology, Fresenius Biotech GmbH, Terumo BCT, TherakosPhotopheresis, TEVA, MiltenyiBiotec GmbH, Clinigen Group Ltd, Sandoz International GmbH, Medac Hematology GmbH, Remedy Informatics, Macropharma, Pierre Fabre Médicament SAS, Takeda, Amgen Oncology GmbH, Kiadispharma, Exem Consulting SA and CHUGAI sanofi-aventis.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Passweg, J., Baldomero, H., Peters, C. et al. Hematopoietic SCT in Europe: data and trends in 2012 with special consideration of pediatric transplantation. Bone Marrow Transplant 49, 744–750 (2014). https://doi.org/10.1038/bmt.2014.55
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.55
This article is cited by
-
Comparison of clinical outcomes between peripheral blood stem cells and peripheral blood stem cells plus bone marrow in myelodysplastic syndrome patients with haploidentical transplantation
Bone Marrow Transplantation (2023)
-
Protection of haematopoietic progenitor cell donors: an updated overview of the European landscape
Bone Marrow Transplantation (2023)
-
Bowel wall thickness is a strong predictor of steroid-refractory acute graft-versus-host disease with gut involvement after allo-HSCT
International Journal of Hematology (2022)
-
Safe transfer of pediatric patients from hematopoietic stem cell transplant unit into the pediatric intensive care unit: views of nurses and physicians
Bone Marrow Transplantation (2022)
-
Long-term outcomes and risk factor analysis of steroid-refractory graft versus host disease after hematopoietic stem cell transplantation
Bone Marrow Transplantation (2021)