Abstract
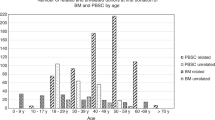
Discrepancies exist between the care of unrelated donors (UDs) and related donors (RDs), particularly regarding medical suitability criteria, consenting procedures and donor follow-up. Changes to the most recent JACIE standards have addressed these issues. We studied 208 RDs who underwent PBSC or BM donation in a single centre during 2004–2013 to determine the impact of regulatory changes on donor care, and assessed the safety and efficacy of stem cell donation in donors not meeting UD medical suitability criteria. We observed significant improvements in donor consenting procedures (P=0.003) and donor follow-up (P=0.007) after stipulations in these areas were introduced. We saw a higher incidence of serious adverse events (SAEs) in RDs not meeting UD suitability criteria (P=0.018), and a higher incidence of SAEs in donors ⩾60 years (P=0.020). Haematopoietic progenitor cell donation is less safe in RDs who do not meet UD criteria for medical suitability. Although changes to JACIE standards have improved practice, development of specific medical suitability for RDs and guidelines around ‘grey areas’ where risks to a donor are unclear or theoretical, will be important in improving RD safety and standardising practice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
O'Donnell PV, Pedersen TL, Confer DL, Rizzo JD, Pulsipher MA, Stroncek D et al. Practice patterns for evaluation, consent, and care of related donors and recipients at hematopoietic cell transplantation centers in the United States. Blood 2010; 115: 5097–5101.
Coluccia P, Crovetti G, Del Fante C, Dallavalle FM, Laszlo D, Ferremi P et al. Screening of related donors and peripheral blood stem cell collection practices at different Italian apheresis centres. Blood Transfus 2012; 10: 440–447.
Clare S, Mank A, Stone R, Davies M, Potting C, Apperley JF et al. Management of related donor care: a European survey. Bone Marrow Transplant 2010; 45: 97–101.
Sacchi N, Costeas P, Hartwell L, Hurley CK, Raffoux C, Rosenmayr A et al. Haematopoietic stem cell donor registries: World Marrow Donor Association recommendations for evaluation of donor health. Bone Marrow Transplant 2008; 42: 9–14.
Halter J, Kodera Y, Ispizua AU, Greinix HT, Schmitz N, Favre G et al. Severe events in donors after allogeneic hematopoietic stem cell donation. Haematologica 2009; 94: 94–101.
WHO Notify Report: Exploring vigilance notification for organs tissues and cells 2011. Available at http://www.notifylibrary.org/sites/default/files/BOOK%20NOTIFY.pdf.
Kodera Y, Yamamoto K, Harada M, Morishima Y, Dohy H, Asano S et al. PBSC collection from family donors in Japan: a prospective survey. Bone Marrow Transplant 2014; 49: 195–200.
Wiersum-Osselton JC, van Walraven SM, Bank I, Lenselink AM, Fibbe WE, van der Bom JG et al. Clinical outcomes after peripheral blood stem cell donation by related donors: a Dutch single-center cohort study. Transfusion 2013; 53: 96–103.
Joint Accreditation Committee—ISCT & EBMT. International Standards for Cellular Therapy Product Collection Processing and Adminstration. 5th edn. Available at http://www.jacie.org Accessed on 9 October 2013.
van Walraven SM, Nicoloso-de Faveri G, Axdorph-Nygell UA, Douglas KW, Jones DA, Lee SJ et al. Family donor care management: principles and recommendations. Bone Marrow Transplant 2010; 45: 1269–1273.
Halter JP, van Walraven SM, Worel N, Bengtsson M, Hagglund H, Nicoloso de Faveri G et al. Allogeneic hematopoietic stem cell donation-standardized assessment of donor outcome data: a consensus statement from the Worldwide Network for Blood and Marrow Transplantation (WBMT). Bone Marrow Transplant 2013; 48: 220–225.
WMDA. International Standards for Unrelated Hematopoietic Progenitor Cell Donor Registries standards W. International Standards for Unrelated Hematopoietic Progenitor Cell Donor Registries. Available at http://www.worldmarrow.org/fileadmin/Committees/STDC/20140101-STDC-WMDA_Standards.pdf. Accessed on 9 October 2013.
Niederwieser D, Gentilini C, Hegenbart U, Lange T, Moosmann P, Ponisch W et al. Transmission of donor illness by stem cell transplantation: should screening be different in older donors? Bone Marrow Transplant 2004; 34: 657–665.
Lysak D, Koristek Z, Gasova Z, Skoumalova I, Jindra P . Efficacy and safety of peripheral blood stem cell collection in elderly donors; does age interfere? J Clin Apher 2011; 26: 9–16.
Richa E, Papari M, Allen J, Martinez G, Wickrema A, Anastasi J et al. Older age but not donor health impairs allogeneic granulocyte colony-stimulating factor (G-CSF) peripheral blood stem cell mobilization. Biol Blood Marrow Transplant 2009; 15: 1394–1399.
Vasu S, Leitman SF, Tisdale JF, Hsieh MM, Childs RW, Barrett AJ et al. Donor demographic and laboratory predictors of allogeneic peripheral blood stem cell mobilization in an ethnically diverse population. Blood 2008; 112: 2092–2100.
de Lavallade H, Ladaique P, Lemarie C, Furst S, Faucher C, Blaise D et al. Older age does not influence allogeneic peripheral blood stem cell mobilization in a donor population of mostly white ethnic origin. Blood 2009; 113: 1868–1869.
Pulsipher M. RDSafe (related donor safety study): a Multi-Institutional Study of HSC Donor Safety and Quality Life by Pulsipher and Switzer. Available at http://www.researchgrantdatabase.com/g/1R01HL085707.
Shaw BE, Ball L, Beksac M, Bengtsson M, Confer D, Diler S et al. Donor safety: the role of the WMDA in ensuring the safety of volunteer unrelated donors: clinical and ethical considerations. Bone Marrow Transplant 2010; 45: 832–838.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author Contributions
CA and BES designed the research study; CA performed the research and analysed the data; CA, MEE, MNP, AM and BES wrote the paper.
Rights and permissions
About this article
Cite this article
Anthias, C., Ethell, M., Potter, M. et al. The impact of improved JACIE standards on the care of related BM and PBSC donors. Bone Marrow Transplant 50, 244–247 (2015). https://doi.org/10.1038/bmt.2014.260
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.260
This article is cited by
-
Severe short-term adverse events in related bone marrow or peripheral blood stem cell donors
International Journal of Hematology (2023)
-
Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022
Bone Marrow Transplantation (2022)
-
Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019
Bone Marrow Transplantation (2019)
-
Providing both autologous and allogeneic hematopoietic stem cell transplants (HSCT) may have a stronger impact on the outcome of autologous HSCT in adult patients than activity levels or implementation of JACIE at Belgian transplant centres
Bone Marrow Transplantation (2019)