Abstract
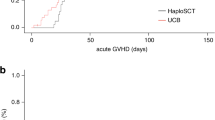
Alternative donor transplantation is increasingly used for high-risk lymphoma patients. We analyzed 1593 transplant recipients (2000–2010) and compared transplant outcomes in recipients of 8/8 allele HLA-A, -B, -C and DRB1 matched unrelated donors (MUDs; n=1176), 7/8 allele HLA mismatched unrelated donors (MMUDs; n=275) and umbilical cord blood donors (1 or 2 units UCB; n=142). Adjusted 3-year non-relapse mortality of MMUD (44%) was higher as compared with MUD (35%; P=0.004), but similar to UCB recipients (37%; P=0.19), although UCB had lower rates of neutrophil and platelet recovery compared with unrelated donor groups. With a median follow-up of 55 months, 3-year adjusted cumulative incidence of relapse was lower after MMUD compared with MUD (25% vs 33%, P=0.003) but similar between UCB and MUD (30% vs 33%; P=0.48). In multivariate analysis, UCB recipients had lower risks of acute and chronic GVHD compared with adult donor groups (UCB vs MUD: hazard ratio (HR)=0.68, P=0.05; HR=0.35; P<0.001). Adjusted 3-year OS was comparable (43% MUD, 37% MMUD and 41% UCB). These data highlight the observation that patients with lymphoma have acceptable survival after alternative donor transplantation. MMUD and UCB can extend the curative potential of allotransplant to patients who lack suitable HLA matched sibling or MUD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Peniket AJ, Ruiz de Elvira MC, Taghipour G, Cordonnier C, Gluckman E, de Witte T et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant 2003; 31: 667–678.
Ratanatharathorn V, Uberti J, Karanes C, Abella E, Lum LG, Momin F et al. Prospective comparative trial of autologous versus allogeneic bone marrow transplantation in patients with non-hodgkin's lymphoma. Blood 1994; 84: 1050–1055.
van Besien K, Carreras J, Bierman PJ, Logan BR, Molina A, King R et al. Unrelated donor hematopoietic cell transplantation for non-hodgkin lymphoma: Long-term outcomes. Biol Blood Marrow Transplant 2009; 15: 554–563.
Lazarus HM, Zhang MJ, Carreras J, Hayes-Lattin BM, Ataergin AS, Bitran JD et al. A comparison of HLA-identical sibling allogeneic versus autologous transplantation for diffuse large B cell lymphoma: a report from the CIBMTR. Biol Blood Marrow Transplant 2010; 16: 35–45.
Khouri IF, McLaughlin P, Saliba RM, Hosing C, Korbling M, Lee MS et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood 2008; 111: 5530–5536.
Khouri IF, Saliba RM, Giralt SA, Lee MS, Okoroji GJ, Hagemeister FB et al. Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortality. Blood 2001; 98: 3595–3599.
Tomblyn M, Brunstein C, Burns LJ, Miller JS, MacMillian M, DeFor TE et al. Similar and promising outcomes in lymphoma patients treated with myeloablative or nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2008; 14: 538–545.
Hale GA, Shrestha S, Le-Rademacher J, Burns LJ, Gibson J, Inwards DJ et al. Alternate donor hematopoietic cell transplantation (HCT) in non-hodgkin lymphoma using lower intensity conditioning: a report from the CIBMTR. Biol Blood Marrow Transplant 2012; 18: 1036–1043.
Rodrigues CA, Rocha V, Dreger P, Brunstein C, Sengeloev H, Finke J et al. Alternative donor hematopoietic stem cell transplantation for mature lymphoid malignancies after reduced-intensity conditioning regimen: similar outcomes with umbilical cord blood and unrelated donor peripheral blood. Haematologica 2014; 99: 370–377.
Sureda A, Robinson S, Canals C, Carella AM, Boogaerts MA, Caballero D et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory hodgkin's lymphoma: an analysis from the lymphoma working party of the european group for blood and marrow transplantation. J Clin Oncol 2008; 26: 455–462.
Anasetti C, Aversa F, Brunstein CG . Back to the future: mismatched unrelated donor, haploidentical related donor, or unrelated umbilical cord blood transplantation? Biol Blood Marrow Transplant 2012; 18: S161–S165.
Brunstein CG, Laughlin MJ . Extending cord blood transplant to adults: dealing with problems and results overall. Semin Hematol 2010; 47: 86–96.
Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood 2007; 110: 4576–4583.
Ballen KK, Klein JP, Pedersen TL, Bhatla D, Duerst R, Kurtzberg J et al. Relationship of race/ethnicity and survival after single umbilical cord blood transplantation for adults and children with leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant 2012; 18: 903–912.
Brunstein CG, Cantero S, Cao Q, Majhail N, McClune B, Burns LJ et al. Promising progression-free survival for patients low and intermediate grade lymphoid malignancies after nonmyeloablative umbilical cord blood transplantation. Biol Blood Marrow Transplant 2009; 15: 214–222.
Majhail NS, Weisdorf DJ, Wagner JE, Defor TE, Brunstein CG, Burns LJ . Comparable results of umbilical cord blood and HLA-matched sibling donor hematopoietic stem cell transplantation after reduced-intensity preparative regimen for advanced hodgkin lymphoma. Blood 2006; 107: 3804–3807.
Marcais A, Porcher R, Robin M, Mohty M, Michalet M, Blaise D et al. Impact of disease status and stem cell source impact on the results of reduced intensity conditioning transplant for hodgkin lymphoma: a retrospective study from the french society of bone marrow graft transplantation and cellular therapy. Haematologica 2013; 98: 1467–1475.
Cutler C, Stevenson K, Kim HT, Brown J, McDonough S, Herrera M et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Bone Marrow Transplant 2011; 46: 659–667.
Devetten MP, Hari PN, Carreras J, Logan BR, van Besien K, Bredeson CN et al. Unrelated donor reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed and refractory hodgkin lymphoma. Biol Blood Marrow Transplant 2009; 15: 109–117.
Avivi I, Canals C, Vernant JP, Wulf G, Nagler A, Hermine O et al. Matched unrelated donor allogeneic transplantation provides comparable long-term outcome to HLA-identical sibling transplantation in relapsed diffuse large B-cell lymphoma. Bone Marrow Transplant 2014; 49: 671–678.
Spellman S, Setterholm M, Maiers M, Noreen H, Oudshoorn M, Fernandez-Viña M et al. Advances in the selection of HLA-compatible donors: refinements in HLA typing and matching over the first 20 years of the National Marrow Donor Program Registry. Biol Blood Marrow Transplant 2008; 14 (9-Suppl): 37–44.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009; 15: 367–369.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P et al. 1995 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Zhang X, Loberiza FR Jr, Klein J, Zhang MJ . A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007; 88: 95–101.
Zhang X, Loberiza FR, Klein JP, Zhang MJ . SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed 2011; 101: 87–93.
Klein JP, Moeschberger ML . Survival Analysis: Techniques for Censored and Truncated Data, 2nd edn, Springer Verlag: New York, USA, 2003.
Hale GA, Shrestha S, Le-Rademacher J, Burns LJ, Gibson J, Inwards DJ et al. Alternate donor hematopoietic cell transplantation (HCT) in non-hodgkin lymphoma using lower intensity conditioning: a report from the CIBMTR. Biol Blood Marrow Transplant 2012; 18: 1036–1043.e1.
Robinson SP, Canals C, Luang JJ, Tilly H, Crawley C, Cahn JY et al. The outcome of reduced intensity allogeneic stem cell transplantation and autologous stem cell transplantation when performed as a first transplant strategy in relapsed follicular lymphoma: an analysis from the lymphoma working party of the EBMT. Bone Marrow Transplant 2013; 48: 1409–1411.
Fernandez-Viña MA, Wang T, Lee SJ, Haagenson M, Aljurf M, Askar M et al. Identification of a permissible HLA mismatch in hematopoietic stem cell transplantation. Blood 2014; 123: 1270–1278.
Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood 2004; 104: 1923–1930.
Pidala J, Wang T, Haagenson M, Spellman SR, Askar M, Battiwalla M et al. Amino acid substitution at peptide-binding pockets of HLA class I molecules increases risk of severe acute GVHD and mortality. Blood 2013; 122: 3651–3658.
Spellman SR, Eapen M, Logan BR, Mueller C, Rubinstein P, Setterholm MI et al. A perspective on the selection of unrelated donors and cord blood units for transplantation. Blood 2012; 120: 259–265.
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics Inc.; *Amgen Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech Inc.; *Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research Inc. Roswell Park Cancer Institute; HistoGenetics Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate members.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bachanova, V., Burns, L., Wang, T. et al. Alternative donors extend transplantation for patients with lymphoma who lack an HLA matched donor. Bone Marrow Transplant 50, 197–203 (2015). https://doi.org/10.1038/bmt.2014.259
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.259
This article is cited by
-
Impact of HLA-mismatched unrelated transplantation in patients with adult T-cell leukemia/lymphoma
Bone Marrow Transplantation (2023)
-
Impact of donor types on reduced-intensity conditioning allogeneic stem cell transplant for mature lymphoid malignancies
Bone Marrow Transplantation (2022)
-
Allogeneic hematopoietic stem cell transplantation from unmanipulated haploidentical donor and unrelated cord blood for T-cell lymphoma: a retrospective study from the Société Francophone de Greffe de Moelle et de Therapie Cellulaire
Bone Marrow Transplantation (2021)
-
Improving outcomes after allogeneic hematopoietic cell transplantation for Hodgkin lymphoma in the brentuximab vedotin era
Bone Marrow Transplantation (2017)
-
Current treatment strategies in relapsed/refractory mantle cell lymphoma: where are we now?
International Journal of Hematology (2017)