Abstract
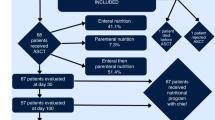
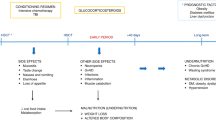
Patients after allogeneic hematopoietic SCT (HSCT) are at risk of malnutrition. To assess the impact of malnutrition after allogeneic HSCT on transplant outcomes, we conducted a retrospective study. Adult patients who received allogeneic HSCT from 2000 to 2009 for standard-risk leukemia and achieved disease-free survival up to 3 months after allogeneic HSCT were included. From participating centers, 145 patients were enrolled. Median age was 46 years (19–68). Patients were classified based on weight loss during 3 months after allogeneic HSCT as follows: normal group (weight loss <5%, n=53), mild malnutrition group (5%⩽weight loss<10%, n=47), severe malnutrition group (10% ⩽weight loss, n=45). The cumulative incidences of 2-year nonrelapse mortality (NRM) were 3.8% in the normal group, 8.5% in the mild malnutrition group and 27.3% in the severe malnutrition group. The probabilities of a 2-year OS were 73.2% in the normal group, 74.5% in the mild malnutrition group and 55.3% in the severe malnutrition group. In multivariate analysis, severe malnutrition was associated with an increased risk of NRM and a worse OS. In conclusion, weight loss ⩾10% was associated with a worse clinical outcome. Prospective studies that identify patients at risk of malnutrition and intervention by a nutritional support team are warranted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. New Engl J Med 2010; 363: 2091–2101.
Lenssen P, Sherry ME, Cheney CL, Nims JW, Sullivan KM, Stern JM et al. Prevalence of nutrition-related problems among long-term survivors of allogeneic marrow transplantation. J Am Diet Assoc 1990; 90: 835–842.
Jacobsohn DA, Margolis J, Doherty J, Anders V, Vogelsang GB . Weight loss and malnutrition in patients with chronic graft-versus-host disease. Bone Marrow Transplant 2002; 29: 231–236.
Urbain P, Birlinger J, Lambert C, Finke J, Bertz H, Biesalski HK . Longitudinal follow-up of nutritional status and its influencing factors in adults undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2013; 48: 446–451.
Lenssen P, Moe GL, Cheney CL, Aker SN, Deeg HJ . Parenteral nutrition in marrow transplant recipients after discharge from the hospital. Exp Hematol 1983; 11: 974–981.
Wu D, Hockenberry DM, Brentnall TA, Baehr PH, Ponec RJ, Kuver R et al. Persistent nausea and anorexia after marrow transplantation: a prospective study of 78 patients. Transplantation 1998; 66: 1319–1324.
Spencer GD, Hackman RC, McDonald GB, Amos DE, Cunningham BA, Meyers JD et al. A prospective study of unexplained nausea and vomiting after marrow transplantation. Transplantation 1986; 42: 602–607.
Charuhas PM, Fosberg KL, Bruemmer B, Aker SN, Leisenring W, Seidel K et al. A double-blind randomized trial comparing outpatient parenteral nutrition with intravenous hydration: effect on resumption of oral intake after marrow transplantation. JPEN J Parenter Enteral Nutr 1997; 21: 157–161.
Fuji S, Takano K, Mori T, Eto T, Taniguchi S, Ohashi K et al. Impact of pretransplant body mass index on the clinical outcome after allogeneic hematopoietic SCT. Bone Marrow Transplant. (e-pub ahead of print 11 August 2014)doi: 10.1038/bmt.2014.178 2014
Navarro WH, Agovi MA, Logan BR, Ballen K, Bolwell BJ, Frangoul H et al. Obesity does not preclude safe and effective myeloablative hematopoietic cell transplantation (HCT) for acute myelogenous leukemia (AML) in adults. Biol Blood Marrow Transplant 2010; 16: 1442–1450.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919.
Kondrup J, Allison SP, Elia M, Vellas B, Plauth M et al. Educational ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003; 22: 415–421.
Kondrup J, Rasmussen HH, Hamberg O, Stanga Z . Ad Hoc EWG nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003; 22: 321–336.
Norman K, Pichard C, Lochs H, Pirlich M . Prognostic impact of disease-related malnutrition. Clin Nutr 2008; 27: 5–15.
Middleton MH, Nazarenko G, Nivison-Smith I, Smerdely P . Prevalence of malnutrition and 12-month incidence of mortality in two Sydney teaching hospitals. Intern Med J 2001; 31: 455–461.
Langer CJ, Hoffman JP, Ottery FD . Clinical significance of weight loss in cancer patients: rationale for the use of anabolic agents in the treatment of cancer-related cachexia. Nutrition 2001; 17: S1–S20.
Pressoir M, Desne S, Berchery D, Rossignol G, Poiree B, Meslier M et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer 2010; 102: 966–971.
Kharfan-Dabaja MA, Chavez JC, Yu DH, Zhu WW, Fernandez-Vertiz EI, Perkins J et al. Severe hypoalbuminemia at day 90 predicts worse nonrelapse mortality and overall survival after allogeneic hematopoietic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant 2011; 17: 384–393.
Kanda Y . Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013; 48: 452–458.
Stratton RJ, Hackston A, Longmore D, Dixon R, Price S, Stroud M et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the 'malnutrition universal screening tool' ('MUST') for adults. Br J Nutr 2004; 92: 799–808.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Johansen N, Kondrup J, Plum LM, Bak L, Norregaard P, Bunch E et al. Effect of nutritional support on clinical outcome in patients at nutritional risk. Clin Nutr 2004; 23: 539–550.
Ravasco P, Monteiro-Grillo I, Vidal PM, Camilo ME . Dietary counseling improves patient outcomes: a prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J Clin Oncol 2005; 23: 1431–1438.
Ravasco P, Monteiro-Grillo I, Camilo M . Individualized nutrition intervention is of major benefit to colorectal cancer patients: long-term follow-up of a randomized controlled trial of nutritional therapy. Am J Clin Nutr 2012; 96: 1346–1353.
Odelli C, Burgess D, Bateman L, Hughes A, Ackland S, Gillies J et al. Nutrition support improves patient outcomes, treatment tolerance and admission characteristics in oesophageal cancer. Clin Oncol (R Coll Radiol) 2005; 17: 639–645.
Glover EI, Phillips SM . Resistance exercise and appropriate nutrition to counteract muscle wasting and promote muscle hypertrophy. Curr Opin Clin Nutr Metab Care 2010; 13: 630–634.
Biolo G, Ciocchi B, Stulle M, Bosutti A, Barazzoni R, Zanetti M et al. Calorie restriction accelerates the catabolism of lean body mass during 2 wk of bed rest. Am J Clin Nutr 2007; 86: 366–372.
Ferrando AA, Stuart CA, Sheffield-Moore M, Wolfe RR . Inactivity amplifies the catabolic response of skeletal muscle to cortisol. J Clin Endocrinol Metab 1999; 84: 3515–3521.
Paddon-Jones D . Interplay of stress and physical inactivity on muscle loss: Nutritional countermeasures. J Nutr 2006; 136: 2123–2126.
Lenssen P, Aker S . Nutrition Support of the Hematopoietic Cell Transplant Recipient. Thomas' Hematopoietic Cell Transplantation. Wiley-Blackwell, Hoboken, NJ, USA, 2009, 1551–1569.
Fuji S, Kim SW, Fukuda T, Kamiya S, Kuwahara S, Takaue Y . Positive impact of maintaining minimal caloric intake above 1.0 x basal energy expenditure on the nutritional status of patients undergoing allogeneic hematopoietic stem cell transplantation. Am J Hematol 2009; 84: 63–64.
Weisdorf SA, Lysne J, Wind D, Haake RJ, Sharp HL, Goldman A et al. Positive effect of prophylactic total parenteral nutrition on long-term outcome of bone marrow transplantation. Transplantation 1987; 43: 833–838.
White JV, Guenter P, Jensen G, Malone A, Schofield M et al. Academy of N consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet 2012; 112: 730–738.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 2014; 123: 3664–3671.
Acknowledgements
We thank the medical, nursing, data-processing, laboratory and clinical staffs at the participating centers for their important contributions to this study and their dedicated care of the patients.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fuji, S., Mori, T., Khattry, N. et al. Severe weight loss in 3 months after allogeneic hematopoietic SCT was associated with an increased risk of subsequent non-relapse mortality. Bone Marrow Transplant 50, 100–105 (2015). https://doi.org/10.1038/bmt.2014.228
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.228
This article is cited by
-
Nutritional status and body mass index before hematopoietic stem cell transplantation (HSCT) and associated outcomes: a rapid review
Supportive Care in Cancer (2024)
-
Bariatric surgery and allogeneic hematopoietic stem cell transplantation: a case series
Bone Marrow Transplantation (2023)
-
Proactive enteral nutrition for patients undergoing allogeneic stem cell transplantation- implementation and clinical outcomes
European Journal of Clinical Nutrition (2023)
-
Nutrition support and clinical outcomes following allogeneic stem cell transplantation
Bone Marrow Transplantation (2023)
-
The association between nutritional risk index and ICU outcomes across hematologic malignancy patients with acute respiratory failure
Annals of Hematology (2023)