Abstract
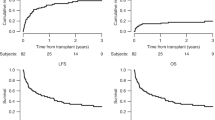
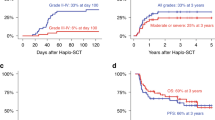
Only 30% of high-risk adult ALL patients in their first complete remission (CR1) are able to receive an HLA-matched sibling stem cell transplant. The role of haploidentical hematopoietic SCT (haplo-HSCT) in post-remission therapy is not well established. Recently, we developed a novel protocol for unmanipulated haploidentical transplantation. In this study, we compared haplo-HSCT with conventional consolidation and maintenance chemotherapy in adult high-risk ALL patients. Between January 2000 and December 2012, 104 patients received conventional chemotherapy and 79 patients received haplo-HSCT. Patients who underwent haplo-HSCT had significantly improved 3-year OS (72.5% vs 26.6%; P<0.001), 3-year disease-free survival (DFS) (63.9% vs 21.1%; P<0.001) and 3-year relapse (18.7% vs 60.5%; P<0.001) rates. The non-relapse mortality (NRM) rate was not different between patients treated with haplo-HSCT vs chemotherapy (19.2% vs 14.4%; P=0.80). In multivariate analysis, the only factor associated with improved OS, better DFS and low risk of relapse was haplo-HSCT. The only factor associated with high NRM was enrollment before 2006. In conclusion, haplo-HSCT may be an option for adults with high-risk ALL in CR1 who do not have an HLA-matched donor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hoelzer D, Thiel E, Loffler H, Buchner T, Ganser A, Heil G et al. Prognostic factors in a multicenter study for treatment of acute lymphoblastic leukemia in adults. Blood 1988; 71: 123–131.
Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood 2005; 106: 3760–3767.
Ottinger HD, Ferencik S, Beelen DW, Lindemann M, Peceny R, Elmaagacli AH et al. Hematopoietic stem cell transplantation: contrasting the outcome of transplantations from HLA-identical siblings, partially HLA-mismatched related donors, and HLA-matched unrelated donors. Blood 2003; 102: 1131–1137.
Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood 2006; 107: 3065–3073.
Xiao-Jun H, Lan-Ping X, Kai-Yan L, Dai-Hong L, Yu W, Huan C et al. Partially matched related donor transplantation can achieve outcomes comparable with unrelated donor transplantation for patients with hematologic malignancies. Clin Cancer Res 2009; 15: 4777–4783.
Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood 2008; 111: 1827–1833.
Chen H, Liu KY, Xu LP, Liu DH, Chen YH, Shi HX et al. Administration of imatinib in the first 90 days after allogeneic hematopoietic cell transplantation in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Chin Med J (Engl) 2011; 124: 246–252.
Liu KY, Chen YH, Liu DH, Xu LP, Huang XJ . A pilot study of low-dose recombinant interleukin-2 for acute lymphoblastic malignancy after unmanipulated allogeneic blood and marrow transplantation. Bone Marrow Transplant 2008; 42: 535–539.
Zhao XS, Yan CH, Liu DH, Xu LP, Liu YR, Liu KY et al. Combined use of WT1 and flow cytometry monitoring can promote sensitivity of predicting relapse after allogeneic HSCT without affecting specificity. Ann Hematol 2013; 92: 1111–1119.
Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood 2012; 119: 3256–3262.
Paulson K, Szwajcer D, Raymond CB, Seftel MD . The role of hematopoietic cell transplantation in adult ALL: clinical equipoise persists. Leuk Res 2014; 38: 176–179.
Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol 2005; 23: 3447–3454.
Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood 2008; 112: 3574–3581.
Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH et al. Haploidentical/mismatched hematopoietic stem cell transplantation without in vitro T cell depletion for T cell acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2012; 18: 716–721.
Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer 2013; 119: 978–985.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W et al. Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant 2009; 15: 257–265.
Mehta J, Singhal S, Gee AP, Chiang KY, Godder K, Rhee Fv F et al. Bone marrow transplantation from partially HLA-mismatched family donors for acute leukemia: single-center experience of 201 patients. Bone Marrow Transplant 2004; 33: 389–396.
Lee S, Chung NG, Cho BS, Eom KS, Kim YJ, Kim HJ et al. Donor-specific differences in long-term outcomes of myeloablative transplantation in adults with Philadelphia-negative acute lymphoblastic leukemia. Leukemia 2010; 24: 2110–2119.
Gupta V, Richards S, Rowe J . Allogeneic, but not autologous, hematopoietic cell transplantation improves survival only among younger adults with acute lymphoblastic leukemia in first remission: an individual patient data meta-analysis. Blood 2013; 121: 339–350.
Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH et al. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transplant 2011; 17: 821–830.
Chen H, Liu KY, Xu LP, Liu DH, Chen YH, Zhao XY et al. Administration of imatinib after allogeneic hematopoietic stem cell transplantation may improve disease-free survival for patients with Philadelphia chromosome-positive acute lymphobla stic leukemia. J Hematol Oncol 2012; 5: 29.
Wang JZ, Liu KY, Xu LP, Liu DH, Han W, Chen H et al. Basiliximab for the treatment of steroid-refractory acute graft-versus-host disease after unmanipulated HLA-mismatched/haploidentical hematopoietic stem cell transplantation. Transplant Proc 2011; 43: 1928–1933.
Anaissie EJ . Diagnosis and therapy of fungal infection in patients with leukemia—new drugs and immunotherapy. Best Pract Res Clin Haematol 2008; 21: 683–690.
Buyukasik Y, Acar K, Kelkitli E, Uz B, Serefhanoglu S, Ozdemir E et al. Hyper-CVAD regimen in routine management of adult acute lymphoblastic leukemia: a retrospective multicenter study. Acta Haematol 2013; 130: 199–205.
Acknowledgements
We thank Editage (www.editage.cn) for their assistance in editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sun, Yq., Wang, J., Jiang, Q. et al. Haploidentical hematopoietic SCT may be superior to conventional consolidation/maintenance chemotherapy as post-remission therapy for high-risk adult ALL. Bone Marrow Transplant 50, 20–25 (2015). https://doi.org/10.1038/bmt.2014.195
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.195
This article is cited by
-
The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China—recommendations from the Chinese Society of Hematology
Journal of Hematology & Oncology (2018)
-
A review of hematopoietic cell transplantation in China: data and trends during 2008–2016
Bone Marrow Transplantation (2017)
-
How do we choose the best donor for T-cell-replete, HLA-haploidentical transplantation?
Journal of Hematology & Oncology (2016)
-
Modern approaches to HLA-haploidentical blood or marrow transplantation
Nature Reviews Clinical Oncology (2016)
-
Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia in Adults
Current Hematologic Malignancy Reports (2016)