Abstract
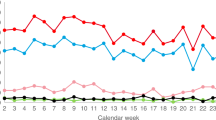
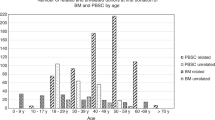
To minimize donor risk and maintain public support, volunteer donor stem cell donation, whether by mobilized leukapheresis or marrow aspiration, requires careful donor eligibility assessment. Many contraindications to stem cell donation exist, yet analyses of donor deferral rates are not available. In a 36-month series encompassing 2493 potential stem cell donors, we analyzed frequencies and reasons for deferrals. All were presumed eligible by their registries because of previously submitted structured health questionnaire and formal telephone interviews. After assessment by our center’s physicians, 3.3% of donors proved ineligible, but 5.6% more were eligible for only one of the collection methods. Higher deferral rates were associated with female sex, increasing age and mobilized stem cell donation vs marrow. Exclusion criteria were identified with approximately similar frequency by medical history, physical examination and laboratory testing. Reasons for deferrals almost exclusively served to protect donor safety; the rare recipient-directed safety concerns could be, and often were, overridden in agreement with the transplant center. As formal analyses have shown, with careful assessment, stem cell donation is acceptably safe, but the plethora of deferral reasons mandate that only physicians with specific experience should evaluate stem cell donors, that is, this task should not be delegated to paramedical personnel.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010; 303: 1617–1624.
Gratwohl A, Baldomero H, Passweg J . Hematopoietic stem cell transplantation activity in Europe. Curr Opin Hematol 2013; 20: 485–493.
Passweg JR, Baldomero H, Bregni M, Cesaro S, Dreger P, Duarte RF et al. Hematopoietic SCT in Europe: data and trends in 2011. Bone Marrow Transplant 2013; 48: 1161–1167.
Stähle M, Müller CH for ZKRD Jahresbericht/Annual Report 2011. Available at: www.zkrd.de/_pdf/Jahresbericht_2011.pdf (last accessed 2 April 2014).
Halter J, Kodera Y, Ispizua AU, Greinix HT, Schmitz N, Favre G et al. Severe events in donors after allogeneic hematopoietic stem cell donation. Haematologica 2009; 94: 94–101.
Holig K, Kramer M, Kroschinsky F, Bornhauser M, Mengling T, Schmidt AH et al. Safety and efficacy of hematopoietic stem cell collection from mobilized peripheral blood in unrelated volunteers: 12 years of single-center experience in 3928 donors. Blood 2009; 114: 3757–3763.
Hölig K . G-CSF in healthy allogeneic stem cell donors. Transf Med Hemother 2013; 40: 225–235.
McCullough J, Kahn J, Adamson J, Anderlini P, Benjamin R, Confer DL et al. Hematopoietic growth factors—use in normal blood and stem cell donors. Transfusion 2008; 48: 2008–2025.
Pulsipher MA, Chitphakdithai P, Miller JP, Logan BR, King RJ, Rizzo JD et al. Adverse events among 2408 unrelated donors of peripheral blood stem cells: results of a prospective trial from the National Marrow Donor Program. Blood 2009; 113: 3604–3611.
Pulsipher MA, Chitphakdithai P, Logan BR, Shaw BE, Wingard JR, Lazarus HM et al. Acute toxicities of unrelated bone marrow versus peripheral blood stem cell donation: results of a prospective trial from the National Marrow Donor Program. Blood 2013; 121: 197–206.
Siegmund-Schultze N . Organspende in Deutschland: Wege aus einer angespannten Situation [Organ donation in Germany: Proposed resolutions for a tense situation]. Deutsches Ärzteblatt 2013; 110: A2118–A2124.
Elger B, Cabrera L . Eth' ways to increase donation of haematopoietic stem cells. Bioethica Forum 2012; 5: 92–99.
Henseler O, Heiden M, Haschberger B, Hesse J, Seitz R . Bericht zur Meldung nach §21TFG für die Jahre 2010 und 2011 [Report of registed data acc. to §21 German Transfusion Act for 2010 and 2011]. Bundesgesundheitsblatt 2013; 56: 1352–1367.
Pathak C, Pujani M, Pahuja S, Jain M . Adverse reactions in whole blood donors: an Indian scenario. Blood Transfus 2011; 9: 46–49.
Crocco I, Franchini M, Garozzo G, Gandini AR, Gandini G, Bonomo P et al. Adverse reactions in blood and apheresis donors: experience from two Italian transfusion centres. Blood Transfus 2009; 7: 35–38.
Amrein K, Valentin A, Lanzer G, Drexler C . Adverse events and safety issues in blood donation—a comprehensive review. Blood Rev 2012; 26: 33–42.
Müller-Steinhardt M, Weidmann C, Wiesneth M, Weck E, Seifried E, Brade J et al. Donor deferral rates after the implementation of a new German blood donor questionnaire. Transf Med Hemother 2012; 39: 17–22.
Zou S, Musavi F, Notari EP, Rios JA, Trouern-Trend J, Fang CT . Donor deferral and resulting donor loss at the American Red Cross Blood Services, 2001 through 2006. Transfusion 2008; 48: 2531–2539.
Coluccia P, Crovetti G, Del FC, Dallavalle FM, Laszlo D, Ferremi P et al. Screening of related donors and peripheral blood stem cell collection practices at different Italian apheresis centres. Blood Transfus 2012; 10: 440–447.
WMDA. WMDA Donor Medical Suitability Recommendations. Available at: http://www.worldmarroworg/donorsuitability/indexphp/Main_Page (last accessed 2 April 2014).
Clark JS, Adler G, Salkic NN, Ciechanowicz A . Allele frequency distribution of 1691G>A F5 (which confers Factor V Leiden) across Europe, including Slavic populations. J Appl Genet 2013; 54: 441–446.
Fairweather D, Frisancho-Kiss S, Rose NR . Sex differences in autoimmune disease from a pathological perspective. Am J Pathol 2008; 173: 600–609.
Anderlini P, Przepiorka D, Korbling M, Champlin R . Blood stem cell procurement: donor safety issues. Bone Marrow Transplant 1998; 21 ( (Suppl 3): ) S35–S39.
Hamilton JA . Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 2008; 8: 533–544.
Kroschinsky F, Hundertmark J, Mauersberger S, Hermes M, Poppe-Thiede K, Rutt C et al. Severe autoimmune hyperthyroidism after donation of growth factor-primed allogeneic peripheral blood progenitor cells. Haematologica 2004; 89: ECR05.
Nasilowska-Adamska B, Perkowska-Ptasinska A, Tomaszewska A, Serwacka A, Marianska B . Acute glomerulonephritis in a donor as a side effect of allogeneic peripheral blood stem cell mobilization with granulocyte colony-stimulating factor. Int J Hematol 2010; 92: 765–768.
Openshaw H, Stuve O, Antel JP, Nash R, Lund BT, Weiner LP et al. Multiple sclerosis flares associated with recombinant granulocyte colony-stimulating factor. Neurology 2000; 54: 2147–2150.
Parkkali T, Volin L, Siren MK, Ruutu T . Acute iritis induced by granulocyte colony-stimulating factor used for mobilization in a volunteer unrelated peripheral blood progenitor cell donor. Bone Marrow Transplant 1996; 17: 433–434.
Shaw BE, Chapman J, Fechter M, Foeken L, Greinix H, Hwang W et al. Towards a global system of vigilance and surveillance in unrelated donors of haematopoietic progenitor cells for transplantation. Bone Marrow Transplant 2013; 48: 1506–1509.
Halter J Donor outcome follow up. Available at: http://www.wbmtorg/en/httpswww.dropboxcomshacirk/ 2013 (last accessed 2 April 2014).
Finke J, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR et al. Prognostic factors affecting outcome after allogeneic transplantation for hematological malignancies from unrelated donors: results from a randomized trial. Biol Blood Marrow Transplant 2012; 18: 1716–1726.
Mueller MM, Bialleck H, Bomke B, Brauninger S, Varga C, Seidl C et al. Safety and efficacy of healthy volunteer stem cell mobilization with filgrastim G-CSF and mobilized stem cell apheresis: results of a prospective longitudinal 5-year follow-up study. Vox Sang 2013; 104: 46–54.
Vasu S, Leitman SF, Tisdale JF, Hsieh MM, Childs RW, Barrett AJ et al. Donor demographic and laboratory predictors of allogeneic peripheral blood stem cell mobilization in an ethnically diverse population. Blood 2008; 112: 2092–2100.
Acknowledgements
The unselfish gift of stem cells by our volunteer donors is humbly acknowledged. ES and HB are members of the LOEWE Cell and Gene Therapy Frankfurt faculty and MS is the recipient of a LOEWE CGT clinician scientist scholarship, funded by Hessian Ministry of Higher Education, Research and the Arts ref.no.: III L 4 518/17.004 (2010/2013). No outside funding was used for these studies.
Author Contributions
KT, SB, BL, KUC, MS and HB were involved in donor evaluation. KT and SB entered and extracted data. BL performed statistical analyses. KT, SB and HB analyzed the data and wrote the manuscript. ES and HB share the overall responsibility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bräuninger, S., Thorausch, K., Luxembourg, B. et al. Deferrals of volunteer stem cell donors referred for evaluation for matched-unrelated stem cell donation. Bone Marrow Transplant 49, 1419–1425 (2014). https://doi.org/10.1038/bmt.2014.171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.171
This article is cited by
-
Superior physical and mental health of healthy volunteers before and five years after mobilized stem cell donation
Journal of Translational Medicine (2022)
-
Basic characteristics and safety of donation in related and unrelated haematopoietic progenitor cell donors – first 10 years of prospective donor follow-up of Swiss donors
Bone Marrow Transplantation (2022)
-
Generation and validation of a formula to calculate hemoglobin loss on a cohort of healthy adults subjected to controlled blood loss
Journal of Translational Medicine (2021)
-
Donor-intrinsic variables determine mobilization efficiency: analyses from a cohort of sixty twice-mobilized stem cell donors
Journal of Translational Medicine (2020)
-
Mobilization of hematopoietic stem cells with the novel CXCR4 antagonist POL6326 (balixafortide) in healthy volunteers—results of a dose escalation trial
Journal of Translational Medicine (2017)