Abstract
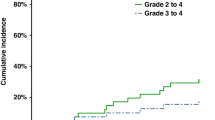
We describe outcomes after allogeneic hematopoietic cell transplantation (HCT) for mycosis fungoides and Sezary syndrome (MF/SS). Outcomes of 129 subjects with MF/SS reported to the Center for the International Blood and Marrow Transplant from 2000–2009. Median time from diagnosis to transplant was 30 (4–206) months and most subjects were with multiply relapsed/ refractory disease. The majority (64%) received non-myeloablative conditioning (NST) or reduced intensity conditioning (RIC). NST/RIC recipients were older in age compared with myeloablative recipients (median age 51 vs 44 years, P=0.005) and transplanted in recent years. Non-relapse mortality (NRM) at 1 and 5 years was 19% (95% confidence interval (CI) 12–27%) and 22% (95% CI 15–31%), respectively. Risk of disease progression was 50% (95% CI 41–60%) at 1 year and 61% (95% CI 50–71%) at 5 years. PFS at 1 and 5 years was 31% (95% CI 22–40%) and 17% (95% CI 9–26%), respectively. OS at 1 and 5 years was 54% (95% CI 45–63%) and 32% (95% CI 22–44%), respectively. Allogeneic HCT in MF/SS results in 5-year survival in approximately one-third of patients and of those, half remain disease-free.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Sausville EA, Eddy JL, Makuch RW, Fischmann AB, Schechter GP, Matthews M et al. Histopathological staging at initial diagnosis of mycosis fungoides and the Sezary syndrome. Definition of three distinctive prognostic groups. Ann Intern Med 1988; 109: 372–382.
Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol 2010; 28: 4730–4739.
Bigler RD, Crilley P, Micaily B, Brady LW, Topolsky D, Bulova S et al. Autologous bone marrow transplantation for advanced stage mycosis fungoides. Bone Marrow Transplant 1991; 7: 133–137.
Olavarria E, Child F, Woolford A, Whittaker SJ, Davis JG, McDonald C et al. T-cell depletion and autologous stem cell transplantation in the management of tumour stage mycosis fungoides with peripheral blood involvement. Br J Haematol 2001; 114: 624–631.
Duarte RF, Schmitz N, Servitje O, Sureda A . Haematopoietic stem cell transplantation for patients with primary cutaneous T-cell lymphoma. Bone Marrow Transplant 2008; 41: 597–604.
Molina A, Zain J, Arber DA, Angelopolou M, O’Donnell M, Murata-Collins J et al. Durable clinical, cytogenetic, and molecular remissions after allogeneic hematopoietic cell transplantation for refractory Sezary syndrome and mycosis fungoides. J Clin Oncol 2005; 23: 6163–6171.
Duarte RF, Canals C, Onida F, Gabriel IH, Arranz R, Arcese W et al. Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and sezary syndrome: a retrospective analysis of the lymphoma working party of the european group for blood and marrow transplantation. J Clin Oncol 2010; 28: 4492–4499.
Lechowicz M, Agovi M, Carreras J, Lazarus HM, Laport GG, Montoto S et al. Allogeneic Hematopoietic Cell Transplantation (AHCT) for Primary Cutaneous T Cell Lymphoma (CTCL): a Center for International Blood and Marrow Transplant Research (CIBMTR) Review. Blood 2010; 116: 2246 (Abstract 364).
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009; 15: 1628–1633.
Herbert KE, Spencer A, Grigg A, Ryan G, McCormack C, Prince HM . Graft-versus-lymphoma effect in refractory cutaneous T-cell lymphoma after reduced-intensity HLA-matched sibling allogeneic stem cell transplantation. Bone Marrow Transplant 2004; 34: 521–525.
Jacobsen ED, Kim HT, Ho VT, Cutler CS, Koreth J, Fisher DC et al. A large single-center experience with allogeneic stem-cell transplantation for peripheral T-cell non-Hodgkin lymphoma and advanced mycosis fungoides/Sezary syndrome. Ann Oncol 2011; 22: 1608–1613.
Wu PA, Kim YH, Lavori PW, Hoppe RT, Stockerl-Goldstein KE . A meta-analysis of patients receiving allogeneic or autologous hematopoietic stem cell transplant in mycosis fungoides and Sézary syndrome. Biol Blood Marrow Transplant 2009; 15: 982–990.
Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007; 110: 1713–1722.
Duvic M, Donato M, Dabaja B, Richmond H, Singh L, Wei W et al. Total Skin electron beam and non-myeloablative allogeneic hematopoietic stem-cell transplantation in advanced mycosis fungoides and Sézary Syndrome. J Clin Oncol 2010; 28: 2365–2372.
De Masson A, Beylot-Barry M, Bouaziz J-D, Peffault de Latour R, Aubin F, Garciaz S et al. Allogeneic stem cell transplantation for advanced cutaneous T-cell lymphomas: a study from the French Society of Bone Marrow Transplantation and French Study Group on Cutaneous Lymphomas. Haematologica 2014; 99: 527–534.
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24 CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement U10 HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from Allos Therapeutics, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;Gentium SpA; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Remedy Informatics; Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; TerumoBCT; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lechowicz, M., Lazarus, H., Carreras, J. et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant 49, 1360–1365 (2014). https://doi.org/10.1038/bmt.2014.161
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.161
This article is cited by
-
Long term outcomes of nonmyeloablative allogeneic stem cell transplantation with TSEB TLI and ATG for Mycosis Fungoides and Sezary Syndrome
Bone Marrow Transplantation (2024)
-
Allogeneic stem cell transplant for treatment of mycosis fungoides and Sezary syndrome: a systematic review and meta-analysis
Bone Marrow Transplantation (2024)
-
Allogeneic hematopoietic stem cell transplantation after mogamulizumab in T-cell lymphoma patients: a retrospective analysis
International Journal of Hematology (2024)
-
Allogeneic transplantation in Cutaneous T-cell Lymphoma: improved outcomes associated with early transplantation and acute graft versus host disease
Bone Marrow Transplantation (2022)
-
Cutaneous T cell lymphoma
Nature Reviews Disease Primers (2021)