Abstract
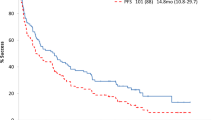
We designed a trial using two sequential cycles of modified high-dose melphalan at 100 mg/m2 and autologous SCT (mHDM/SCT) in AL amyloidosis (light-chain amyloidosis, AL), AL with myeloma (ALM) and host-based high-risk myeloma (hM) patients through SWOG-0115. The primary objective was to evaluate OS. From 2004 to 2010, 93 eligible patients were enrolled at 17 centers in the United States (59 with AL, 9 with ALM and 25 with hM). The median OS for patients with AL and ALM was 68 months and 47 months, respectively, and has not been reached for patients with hM. The median PFS for patients with AL and ALM was 38 months and 16 months, respectively, and has not been reached for patients with hM. The treatment-related mortality (TRM) was 12% (11/93) and was observed only in patients with AL after SCT. Grade 3 and higher non-hematologic adverse events were experienced by 81%, 67% and 57% of patients with AL, ALM and hM, respectively, during the first and second HDM/SCT. This experience demonstrates that with careful selection of patients and use of mHDM for SCT in patients with AL, ALM and hM, even in the setting of a multicenter study, OS can be improved with acceptable TRM and morbidity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Merlini G, Bellotti V . Molecular mechanisms of amyloidosis. N Engl J Med 2003; 349: 583–596.
Kyle RA, Linos A, Beard CM, Linke RP, Gertz MA, O’Fallon WM et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood 1992; 79: 1817–1822.
Comenzo RL, Vosburgh E, Simms RW, Bergethon P, Sarnacki D, Finn K et al. Dose-intensive melphalan with blood stem cell support for the treatment of AL amyloidosis: one-year follow-up in five patients. Blood 1996; 88: 2801–2806.
Cibeira MT, Sanchorawala V, Seldin DC, Quillen K, Berk JL, Dember LM et al. Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood 2011; 118: 4346–4352.
Sanchorawala V . Role of high-dose melphalan and autologous peripheral blood stem cell transplantation in AL amyloidosis. Am J Blood Res 2012; 2: 9–17.
Jaccard A, Moreau P, Leblond V, Leleu X, Benboubker L, Hermine O et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med 2007; 357: 1083–1093.
Gertz MA, Lacy MQ, Dispenzieri A, Kumar SK, Dingli D, Leung N et al. Refinement in patient selection to reduce treatment-related mortality from autologous stem cell transplantation in amyloidosis. Bone Marrow Transplant 2013; 48: 557–561.
Tsai SB, Seldin DC, Quillen K, Berk JL, Ruberg FL, Meier-Ewert H et al. High-dose melphalan and stem cell transplantation for patients with AL amyloidosis: trends in treatment-related mortality over the past 17 years at a single referral center. Blood 2012; 120: 4445–4446.
Sanchorawala V, Skinner M, Quillen K, Finn KT, Doros G, Seldin DC . Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem-cell transplantation. Blood 2007; 110: 3561–3563.
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335: 91–97.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–1883.
Badros A, Barlogie B, Siegel E, Morris C, Desikan R, Zangari M et al. Autologous stem cell transplantation in elderly multiple myeloma patients over the age of 70 years. Br J Haematol 2001; 114: 600–607.
Siegel DS, Desikan KR, Mehta J, Singhal S, Fassas A, Munshi N et al. Age is not a prognostic variable with autotransplants for multiple myeloma. Blood 1999; 93: 51–54.
Seldin DC, Anderson JJ, Skinner M, Malek K, Wright DG, Quillen K et al. Successful treatment of AL amyloidosis with high-dose melphalan and autologous stem cell transplantation in patients over age 65. Blood 2006; 108: 3945–3947.
Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol 2005; 79: 319–328.
Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol 1998; 102: 1115–1123.
Berenson JR, Crowley JJ, Grogan TM, Zangmeister J, Briggs AD, Mills GM et al. Maintenance therapy with alternate-day prednisone improves survival in multiple myeloma patients. Blood 2002; 99: 3163–3168.
Dhodapkar MV, Hussein MA, Rasmussen E, Solomon A, Larson RA, Crowley JJ et al. Clinical efficacy of high-dose dexamethasone with maintenance dexamethasone/alpha interferon in patients with primary systemic amyloidosis: results of United States Intergroup Trial Southwest Oncology Group (SWOG) S9628. Blood 2004; 104: 3520–3526.
Comenzo RL, Sanchorawala V, Fisher C, Akpek G, Farhat M, Cerda S et al. Intermediate-dose intravenous melphalan and blood stem cells mobilized with sequential GM+G-CSF or G-CSF alone to treat AL (amyloid light chain) amyloidosis. Br J Haematol 1999; 104: 553–559.
Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet 2007; 370: 1209–1218.
Palumbo A, Triolo S, Argentino C, Bringhen S, Dominietto A, Rus C et al. Dose-intensive melphalan with stem cell support (MEL100) is superior to standard treatment in elderly myeloma patients. Blood 1999; 94: 1248–1253.
Palumbo A, Bringhen S, Bertola A, Cavallo F, Falco P, Massaia M et al. Multiple myeloma: comparison of two dose-intensive melphalan regimens (100 vs 200 mg/m(2)). Leukemia 2004; 18: 133–138.
McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366: 1770–1781.
Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366: 1782–1791.
Acknowledgements
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA37981, CA76448, CA35431, CA20319, CA76132, CA04919, CA46441, CA14028, CA58861, CA58416, CA12644, CA22433, CA46368, CA45377, CA35090 and CA46113.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
VS received research funding from Celgene, Onyx and Millennium; DCS received research funding from Celgene; and RZO received research funding from Celgene, Onyx, Johnson and Johnson and Millennium. Other authors declare no conflict of interest.
Additional information
Author contributions
VS: concept and design, data analysis and interpretation, manuscript writing; AH: concept and design, collection and assembly of data, data analysis and interpretation, final approval of manuscript; DCS: data analysis and interpretation, final approval of manuscript; KTF: collection and assembly of data, data analysis and interpretation, final approval of manuscript; SAF: collection and assembly of data, data analysis and interpretation, final approval of manuscript; RS: concept and design, collection and assembly of data, data analysis and interpretation, concept and design, data analysis and interpretation; BM: final approval of manuscript; HFS: final approval of manuscript; LAH: final approval of manuscript; RMD: final approval of manuscript; ROZ: final approval of manuscript; and BB: concept and design, data analysis and interpretation, final approval of manuscript.
Rights and permissions
About this article
Cite this article
Sanchorawala, V., Hoering, A., Seldin, D. et al. Modified high-dose melphalan and autologous SCT for AL amyloidosis or high-risk myeloma: analysis of SWOG trial S0115. Bone Marrow Transplant 48, 1537–1542 (2013). https://doi.org/10.1038/bmt.2013.98
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.98
Keywords
This article is cited by
-
High-dose melphalan and autologous peripheral blood stem cell transplantation in patients with AL amyloidosis and cardiac defibrillators
Bone Marrow Transplantation (2019)
-
Auto-SCT improves survival in systemic light chain amyloidosis: a retrospective analysis with 14-year follow-up
Bone Marrow Transplantation (2014)