Abstract
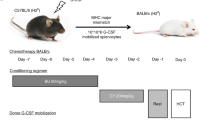
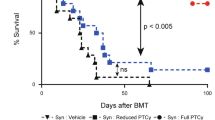
Allogeneic hematopoietic SCT (HSCT) has been shown to be an effective treatment option for advanced renal cell cancer (RCC). However, tumor resistance/relapse remains as the main post transplant issue. Therefore, enhancing graft-versus-tumor (GVT) activity without increasing GVHD is critical for improving the outcome of HSCT. We explored the GVT effect of haploidentical-SCT (haplo-SCT) against RCC in murine models. Lethally irradiated CB6F1 (H2Kb/d) recipients were transplanted with T-cell-depleted BM cells from B6CBAF1 (H2Kb/k) mice. Haplo-SCT combined with a low-dose haploidentical (HI) T-cell infusion (1 × 105) successfully provided GVT activity without incurring GVHD. This effect elicited murine RCC growth control and consequently displayed a comparative survival advantage of haplo-SCT recipients when compared with MHC-matched (B6D2F1→CB6F1) and parent-F1 (B6→CB6F1) transplant recipients. Recipients of haplo-SCT had an increase in donor-derived splenic T-cell numbers, T-cell proliferation and IFN-γ-secreting donor-derived T-cells, a critical aspect for anti-tumor activity. The splenocytes from B6CBAF1 mice had a higher cytotoxicity against RENCA cells than the splenocytes from B6 and B6D2F1 donors after tumor challenge. These findings suggest that haplo-SCT might be an innovative immunotherapeutic platform for solid tumors, particularly for renal cell carcinoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Childs R, Chernoff A, Contentin N, Bahceci E, Schrump D, Leitman S et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med 2000; 343: 750–758.
Tykodi SS, Warren EH, Thompson JA, Riddell SR, Childs RW, Otterud BE et al. Allogeneic hematopoietic cell transplantation for metastatic renal cell carcinoma after nonmyeloablative conditioning: toxicity, clinical response, and immunological response to minor histocompatibility antigens. Clin Cancer Res 2004; 10: 7799–7811.
Barkholt L, Bregni M, Remberger M, Blaise D, Peccatori J, Massenkeil G et al. Allogeneic haematopoietic stem cell transplantation for metastatic renal carcinoma in Europe. Ann Oncol 2006; 17: 1134–1140.
Ueno NT, Childs RW . What’s past is prologue: lessons learned and the need for further development of allogeneic hematopoietic stem cell transplantation for renal cell carcinoma. Biol Blood Marrow Transplant 2007; 13: 31–33.
Takahashi Y, Harashima N, Kajigaya S, Yokoyama H, Cherkasova E, McCoy JP et al. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J Clin Invest 2008; 118: 1099–1109.
Rini BI, Halabi S, Barrier R, Margolin KA, Avigan D, Logan T et al. Adoptive immunotherapy by allogeneic stem cell transplantation for metastatic renal cell carcinoma: a CALGB intergroup phase II study. Biol Blood Marrow Transplant 2006; 12: 778–785.
Bregni M, Bernardi M, Servida P, Pescarollo A, Crocchiolo R, Treppiedi E et al. Long-term follow-up of metastatic renal cancer patients undergoing reduced-intensity allografting. Bone Marrow Transplant 2009; 44: 237–242.
Vanclee A, van Gelder M, Schouten HC, Bos GM . Graft-versus-tumor effects on murine mammary carcinoma in a model of nonmyeloablative haploidentical stem cell transplantation. Bone Marrow Transplant 2006; 37: 1043–1049.
Satoh M, Miyamura K, Yamada M, Ishidoya S, Childs RW, Arai Y . Haploidentical, non-myeloablative stem-cell transplantation for advanced renal-cell carcinoma. Lancet Oncol 2004; 5: 125–126.
Perez-Martinez A, Leung W, Munoz E, Iyengar R, Ramirez M, Vicario JL et al. KIR-HLA receptor-ligand mismatch associated with a graft-versus-tumor effect in haploidentical stem cell transplantation for pediatric metastatic solid tumors. Pediatr Blood Cancer 2009; 53: 120–124.
Perez-Martinez A, de Prada Vicente I, Fernandez L, Gonzalez-Vicent M, Valentin J, Martin R et al. Natural killer cells can exert a graft-vs-tumor effect in haploidentical stem cell transplantation for pediatric solid tumors. Exp Hematol 2012; 40: 882–891 e1.
Lang P, Pfeiffer M, Muller I, Schumm M, Ebinger M, Koscielniak E et al. Haploidentical stem cell transplantation in patients with pediatric solid tumors: preliminary results of a pilot study and analysis of graft versus tumor effects. Klin Padiatr 2006; 218: 321–326.
Ramirez-Montagut T, Chow A, Kochman AA, Smith OM, Suh D, Sindhi H et al. IFN-gamma and Fas ligand are required for graft-versus-tumor activity against renal cell carcinoma in the absence of lethal graft-versus-host disease. J Immunol 2007; 179: 1669–1680.
Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J, Crawford JM et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood 1996; 88: 3230–3239.
Chagnon F, Tanguay S, Ozdal OL, Guan M, Ozen ZZ, Ripeau JS et al. Potentiation of a dendritic cell vaccine for murine renal cell carcinoma by CpG oligonucleotides. Clin Cancer Res 2005; 11: 1302–1311.
Sayers TJ, Brooks AD, Seki N, Smyth MJ, Yagita H, Blazar BR et al. T cell lysis of murine renal cancer: multiple signaling pathways for cell death via Fas. J Leukoc Biol 2000; 68: 81–86.
Igarashi T, Wynberg J, Srinivasan R, Becknell B, McCoy JP, Takahashi Y et al. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood 2004; 104: 170–177.
Lundqvist A, McCoy JP, Samsel L, Childs R . Reduction of GVHD and enhanced antitumor effects after adoptive infusion of alloreactive Ly49-mismatched NK cells from MHC-matched donors. Blood 2007; 109: 3603–3606.
Eto M, Harano M, Tatsugami K, Harada M, Kamiryo Y, Kiyoshima K et al. Cyclophosphamide-using nonmyeloablative allogeneic cell therapy against renal cancer with a reduced risk of graft-versus-host disease. Clin Cancer Res 2007; 13: 1029–1035.
Eto M, Kamiryo Y, Takeuchi A, Harano M, Tatsugami K, Harada M et al. Posttransplant administration of cyclophosphamide and donor lymphocyte infusion induces potent antitumor immunity to solid tumor. Clin Cancer Res 2008; 14: 2833–2840.
Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008; 14: 641–650.
Han Y, Yu J, Cao S, Li H, Ren B, An X et al. Fetal-maternal microchimerism enhances the survival effect of interleukin-2-activated haploidentical peripheral blood stem cell treatment in patients with advanced solid cancer. Cancer Biother Radiopharm 2010; 25: 741–746.
Re F, Staudacher C, Zamai L, Vecchio V, Bregni M . Killer cell Ig-like receptors ligand-mismatched, alloreactive natural killer cells lyse primary solid tumors. Cancer 2006; 107: 640–648.
Passweg JR, Koehl U, Uharek L, Meyer-Monard S, Tichelli A . Natural-killer-cell-based treatment in haematopoietic stem-cell transplantation. Best Pract Res Clin Haematol 2006; 19: 811–824.
Singer EA, Gupta GN, Srinivasan R . Targeted therapeutic strategies for the management of renal cell carcinoma. Curr Opin Oncol 2012; 24: 284–290.
Coppin C, Kollmannsberger C, Le L, Porzsolt F, Wilt TJ . Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int 2011; 108: 1556–1563.
Pedrazzoli P, Comoli P, Montagna D, Demirer T, Bregni M, Ebmt S . Is adoptive T-cell therapy for solid tumors coming of age? Bone Marrow Transplant 2012; 47: 1013–1019.
Tykodi SS, Voong LN, Warren EH . Combining allogeneic immunotherapy with an mTOR inhibitor for advanced renal cell carcinoma. Bone Marrow Transplant 2010; 45: 1360–1362.
Bregni M, Vitali G, Peccatori J . Combining allografting with mTOR inhibitors for metastatic renal cell cancer. Bone Marrow Transplant 2011; 46: 1586.
Bregni M, Herr W, Blaise D . Solid Tumor Working Party of E. Allogeneic stem cell transplantation for renal cell carcinoma. Expert Rev Anticancer Ther 2011; 11: 901–911.
Choi MS, Lim JY, Cho BS, Kim YJ, Chung NG, Jeong DC et al. The role of regulatory T cells during the attenuation of graft-versus-leukemia activity following donor leukocyte infusion in mice. Leuk Res 2011; 35: 1549–1556.
Acknowledgements
This study is supported by American Cancer Society.
Author contributions: TBA contributed to the designing of the experiments, acquiring and analyzing the data and writing the manuscript. CTS, CPB, CSB, MMP and SC acquired the data, conducted animal experiments and analyzed the data. NF contributed to the designing of the experiments and supervised the project. OA had the concept, designed the experiments, interpreted the data, supervised all the experiments and revised the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Budak-Alpdogan, T., Sauter, C., Bailey, C. et al. Haploidentical hematopoietic SCT increases graft-versus-tumor effect against renal cell carcinoma. Bone Marrow Transplant 48, 1084–1090 (2013). https://doi.org/10.1038/bmt.2013.9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.9