Abstract
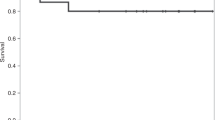
Success of umbilical cord blood transplantation (UCBT) has been limited by a high rate of graft failure and delayed hematological recovery. It has been postulated that MSCs have hematopoiesis-supportive properties. Therefore, to overcome the limitation of UCBT, third-party UCB-derived MSCs were co-transplanted in recipients receiving unrelated UCBT. Seven patients received UCB and third-party UCB–MSCs. Hematopoietic recovery and transplantation outcomes were compared with historic controls. There was no acute toxicity associated with the infusion of MSCs. The median day to neutrophil engraftment was 19 days in patients, as compared with 24 days in controls (P=0.03). The median day of platelet engraftment was 47 days and 57 days in patients and controls, respectively (P=0.26). In addition, there was no engraftment failure in the MSC group. The incidence of acute and chronic GVHD was comparable between the two groups. However, veno-occlusive disease and TRM did not occur in the MSC group. Third-party UCB–MSCs infusion was safe and feasible. MSCs may also enhance the engraftment of UCBT and prevent rejection. In addition, MSCs may have a role in decreasing TRM. Randomized, controlled trials are required to confirm these results and longer follow-up will determine the effects of MSCs on the risk of relapse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Peters C, Cornish JM, Parikh SH, Kurtzberg J . Stem cell source and outcome after hematopoietic stem cell transplantation (HSCT) in children and adolescents with acute leukemia. Pediatr Clin North Am 2010; 57: 27–46.
Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID . Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med 2010; 16: 232–236.
de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med 2012; 367: 2305–2315.
Ding DC, Shyu WC, Lin SZ . Mesenchymal stem cells. Cell Transplant 2011; 20: 5–14.
Siegel G, Schafer R, Dazzi F . The immunosuppressive properties of mesenchymal stem cells. Transplantation 2009; 87: S45–S49.
Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005; 105: 2214–2219.
Aggarwal S, Pittenger MF . Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815–1822.
Le Blanc K, Samuelsson H, Gustafsson B, Remberger M, Sundberg B, Arvidson J et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia 2007; 21: 1733–1738.
Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 2007; 110: 2764–2767.
Chan WK, Lau AS, Li JC, Law HK, Lau YL, Chan GC . MHC expression kinetics and immunogenicity of mesenchymal stromal cells after short-term IFN-gamma challenge. Exp Hematol 2008; 36: 1545–1555.
Francois M, Romieu-Mourez R, Stock-Martineau S, Boivin MN, Bramson JL, Galipeau J . Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood 2009; 114: 2632–2638.
Romieu-Mourez R, Francois M, Boivin MN, Bouchentouf M, Spaner DE, Galipeau J . Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol 2009; 182: 7963–7973.
Le Blanc K, Fibbe W, Frassoni F, Locatelli F, Ringden O . Mesenchymal stem cells for acute graft-versus-host disease—Reply. Lancet 2008; 372: 716–716.
Chen L, Tredget EE, Liu C, Wu Y . Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PLoS One 2009; 4: e7119.
Wu KH, Chan CK, Tsai C, Chang YH, Sieber M, Chiu TH et al. Effective treatment of severe steroid-resistant acute graft-versus-host disease with umbilical cord-derived mesenchymal stem cells. Transplantation 2011; 91: 1412–1416.
Mougiakakos D, Machaczka M, Jitschin R, Klimkowska M, Entesarian M, Bryceson YT et al. Treatment of familial hemophagocytic lymphohistiocytosis with third-party mesenchymal stromal cells. Stem Cells Dev 2012; 21: 3147–3151.
Yang SE, Ha CW, Jung M, Jin HJ, Lee M, Song H et al. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy 2004; 6: 476–486.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Kim DH, Yoo KH, Yim YS, Choi J, Lee SH, Jung HL et al. Cotransplanted bone marrow derived mesenchymal stem cells (MSC) enhanced engraftment of hematopoietic stem cells in a MSC-dose dependent manner in NOD/SCID mice. J Korean Med Sci 2006; 21: 1000–1004.
Noort WA, Kruisselbrink AB, in't Anker PS, Kruger M, van Bezooijen RL, de Paus RA et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol 2002; 30: 870–878.
Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18: 307–316.
Mueller SM, Glowacki J . Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem 2001; 82: 583–590.
Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE . Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab 1999; 17: 171–177.
Stenderup K, Justesen J, Clausen C, Kassem M . Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 2003; 33: 919–926.
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K . Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006; 24: 1294–1301.
Fan CG, Zhang QJ, Zhou JR . Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev 2011; 7: 195–207.
Huang GP, Pan ZJ, Jia BB, Zheng Q, Xie CG, Gu JH et al. Ex vivo expansion and transplantation of hematopoietic stem/progenitor cells supported by mesenchymal stem cells from human umbilical cord blood. Cell Transplant 2007; 16: 579–585.
Almeida-Porada G, Porada CD, Tran N, Zanjani ED . Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood 2000; 95: 3620–3627.
Friedman R, Betancur M, Boissel L, Tuncer H, Cetrulo C, Klingemann H . Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation. Biol Blood Marrow Transplant 2007; 13: 1477–1486.
Kimura T, Asada R, Wang J, Morioka M, Matsui K, Kobayashi K et al. Identification of long-term repopulating potential of human cord blood-derived CD34-flt3- severe combined immunodeficiency-repopulating cells by intra-bone marrow injection. Stem Cells 2007; 25: 1348–1355.
Muguruma Y, Yahata T, Miyatake H, Sato T, Uno T, Itoh J et al. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood 2006; 107: 1878–1887.
Pereira RF, Halford KW, O'Hara MD, Leeper DB, Sokolov BP, Pollard MD et al. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci USA 1995; 92: 4857–4861.
Bensidhoum M, Chapel A, Francois S, Demarquay C, Mazurier C, Fouillard L et al. Homing of in vitro expanded Stro-1− or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood 2004; 103: 3313–3319.
Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol 2001; 29: 244–255.
Horwitz EM, Dominici M . How do mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy 2008; 10: 771–774.
Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE . Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 2006; 24: 1030–1041.
Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL . Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res 2000; 9: 841–848.
Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL . Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol 1998; 176: 57–66.
Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest 2000; 106: 1331–1339.
Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 2005; 11: 389–398.
Macmillan ML, Blazar BR, DeFor TE, Wagner JE . Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: results of a phase I-II clinical trial. Bone Marrow Transplant 2009; 43: 447–454.
Bernardo ME, Ball LM, Cometa AM, Roelofs H, Zecca M, Avanzini MA et al. Co-infusion of ex vivo-expanded, parental MSCs prevents life-threatening acute GVHD, but does not reduce the risk of graft failure in pediatric patients undergoing allogeneic umbilical cord blood transplantation. Bone Marrow Transplant 2011; 46: 200–207.
Prockop DJ, Olson SD . Clinical trials with adult stem/progenitor cells for tissue repair: let's not overlook some essential precautions. Blood 2007; 109: 3147–3151.
Bang OY, Lee JS, Lee PH, Lee G . Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 2005; 57: 874–882.
Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J 2009; 30: 2722–2732.
Perasso L, Cogo CE, Giunti D, Gandolfo C, Ruggeri P, Uccelli A et al. Systemic administration of mesenchymal stem cells increases neuron survival after global cerebral ischemia in vivo (2VO). Neural Plast 2010; 2010: 534925.
Hu KX, Sun QY, Guo M, Ai HS . The radiation protection and therapy effects of mesenchymal stem cells in mice with acute radiation injury. Br J Radiol 2010; 83: 52–58.
Soloviev A, Prudnikov I, Tsyvkin V, Tishkin S, Kyrychenko S, Zelensky S et al. Electrophysiological and contractile evidence of the ability of human mesenchymal stromal cells to correct vascular malfunction in rats after ionizing irradiation. J Physiol Sci 2010; 60: 161–172.
Loffredo FS, Steinhauser ML, Gannon J, Lee RT . Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell 2011; 8: 389–398.
Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AA et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res 2011; 6: 206–214.
Ringden O, Uzunel M, Sundberg B, Lonnies L, Nava S, Gustafsson J et al. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia 2007; 21: 2271–2276.
Grinnemo KH, Mansson-Broberg A, Leblanc K, Corbascio M, Wardell E, Siddiqui AJ et al. Human mesenchymal stem cells do not differentiate into cardiomyocytes in a cardiac ischemic xenomodel. Ann Med 2006; 38: 144–153.
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008; 371: 1579–1586.
Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G et al. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant 2011; 17: 534–541.
Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB . Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One 2010; 5: e9016.
Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D . Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004; 103: 4619–4621.
Acknowledgements
This study was supported by a grant from the National R&D Program Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (0520290), and by a grant from the Korea Health 21R&D Project, Ministry of Health, Welfare and Family affairs, Republic of Korea (0405-DB00-0101-0016). This paper was presented at the 2011 BMT tandem meeting in Hawaii.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
MWL, KHY, HHK, SJC, WO and YSY designed the research; SJC, DSK and WO performed the cell culture and expansion; SHL, MHS, HC and KWS performed the clinical trial; SHL and MWL collected and analyzed the data; SHL, MWL, KHY, KWS, HHK, MHS, HC and YSY interpreted and discussed the data; and SHL wrote the manuscript.
Rights and permissions
About this article
Cite this article
Lee, S., Lee, M., Yoo, K. et al. Co-transplantation of third-party umbilical cord blood-derived MSCs promotes engraftment in children undergoing unrelated umbilical cord blood transplantation. Bone Marrow Transplant 48, 1040–1045 (2013). https://doi.org/10.1038/bmt.2013.7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.7
Keywords
This article is cited by
-
Current status of clinical trials assessing mesenchymal stem cell therapy for graft versus host disease: a systematic review
Stem Cell Research & Therapy (2022)
-
Efficacy and safety of mesenchymal stem cells co-infusion in allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis
Stem Cell Research & Therapy (2021)
-
Infusion of Mesenchymal Stem Cells to Treat Graft Versus Host Disease: the Role of HLA-G and the Impact of its Polymorphisms
Stem Cell Reviews and Reports (2020)
-
Efficacy and safety of mesenchymal stromal cells for the prophylaxis of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: a meta-analysis of randomized controlled trials
Annals of Hematology (2018)
-
Generation of mesenchymal stromal cells from cord blood: evaluation of in vitro quality parameters prior to clinical use
Stem Cell Research & Therapy (2017)