Abstract
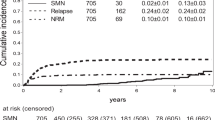
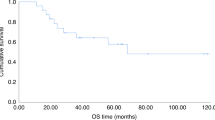
The aim of this study was to determine whether parameters related to TBI impacted upon OS and relapse in patients with acute leukemia in CR who underwent haematopoietic SCT (HSCT) in 11 Italian Radiation Oncology Centres. Data were analysed from 507 patients (313 males; 194 females; median age 15 years; 318 with ALL; 188 with AML; 1 case not recorded). Besides 128 autologous transplants, donors included 192 matched siblings, 74 mismatched family members and 113 unrelated individuals. Autologous and allogeneic transplants were analysed separately. Median follow-up was 40.1 months. TBI schedules and HSCT type were closely related. Uni- and multi-variate analyses showed no parameter was significant for OS or relapse in autologous transplantation. Multivariate analysis showed type of transplant and disease impacted significantly on OS in allogeneic transplantation. Disease, GVHD and TBI dose were risk factors for relapse. This analysis illustrates that Italian Transplant Centre use of TBI is in line with international practice. Most Centres adopted a hyperfractionated schedule that is used worldwide (12 Gy in six fractions over 3 days), which appears to have become standard. TBI doses impacted significantly upon relapse rates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hagenbeek A, Martens ACM . The effect of fractionated versus unfractionated total body irradiation on the growth of the BN acute myelocytic leukemia. Int J Radiat Oncol Biol Phys 1981; 7: 1075–1079.
Gale RP, Butturini A, Bortin MM . What does total body irradiation do in bone marrow transplants for leukemia? Int J Radiat Oncol Biol Phys 1991; 20: 631–634.
Shank B . Total body irradiation. In: Leibel S, Phillips T (eds). Textbook of Radiation Oncology. W.B. Saunders Company: Philadelphia, 1998, 253–275.
Vriesendorp HM . Prediction of effects of therapeutic total body irradiation in man. Radiother Oncol 1990 Suppl 1: 37–50.
Cosset J-M, Socie G, Dubray B, Girinsky T, Fourquet A, Gluckman E . Single dose versus fractionated total body irradiation before bone marrow transplantation: radiobiological and clinical considerations. Int J Radiat Oncol Biol Phys 1994; 30: 477–492.
Cosset JM, Socié G, Girinsky T, Dubray B, Fourquet A, Gluckman E . Radiobiological and clinical bases for total body irradiation in the leukemias and lymphomas. Semin Radiat Oncol 1995; 5: 301–315.
Hill-Kayser CE, Plastaras JP, Tochner Z, Glatstein E . TBI during BM and SCT: review of the past, discussion of the present and consideration of future direction. Bone Marrow Transplant 2011; 46: 475–484.
Gray RJ . A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154.
Scrucca L, Santucci A, Aversa F . Competing risks analysis using R: an easy guide for clinicians. Bone Marrow Transplant 2007; 40: 381–387.
Shank B, O’Reilly R, Cunningham I, Kernan N, Yaholom J, Brochstein J et al. Total body irradiation for bone marrow transplantation: the Memorial Sloan-Kettering Cancer Center experience. Radiother Oncol 1990 Suppl 1: 68–81.
Cox DR . Regression models and life-tables. J R Stat Soc [B] 1972; 34: 187–202.
Thomas ED . Total body irradiation regimens for marrow grafting. Int J Radiat Oncol Biol Phys 1990; 19: 1285–1288.
Thomas ED, Clift RA, Hersman J, Sanders JE, Stewart P, Buckner CD et al. Marrow transplantation for acute nonlymphoblastic leukemia in first remission using fractionated or single-dose irradiation. Int J Radiat Oncol Biol Phys 1982; 8: 817–821.
Shank B, Andreeff M, Li D . Cell survival kinetics in peripheral blood and bone marrow during total body irradiation for marrow transplantation. Int J Radiat Oncol Biol Phys 1983; 9: 1613–1623.
Aristei C, Aversa F, Raymondi C, Marsella AR, Panizza BM, Perrucci E et al. Allogeneic matched T-cell-depleted bone marrow transplantation for acute leukemia patients. Cancer J Sci Am 1996; 2: 330–334.
Aversa F, Terenzi A, Carotti A, Felicini R, Jacucci R, Zei T et al. Improved outcome with T-cell depleted bone marrow transplantation for acute leukemia. J Clin Oncol 1999; 17: 1545–1550.
Terenzi A, Aristei C, Aversa F, Perruccio K, Chionne F, Raymondi C et al. Efficacy of fludarabine as an immunosuppressor for bone marrow transplantation conditioning: preliminary results. Transplant Proc 1996; 28: 3101.
Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socié G, Travis LB et al. Solid cancers after bone marrow transplantation. N Engl J Med 1997; 336: 897–904.
Corvò R, Lamparelli T, Bruno B, Barra S, Van Lint MT, Vitale V et al. Low-dose fractionated total body irradiation (TBI) adversely affects prognosis of patients with leukemia receiving an HLA-matched allogeneic bone marrow transplant from an unrelated donor (UD-BMT). Bone Marrow Transplant 2002; 30: 717–723.
Storb R, Raff RF, Appelbaum FR, Graham TC, Schuening FG, Sale G et al. Comparison of fractionated to single-dose total body irradiation in conditioning canine littermates for DLA-identical marrow grafts. Blood 1989; 74: 1139–1143.
Down JD, Tarbell NJ, Thames HD, Mauch PM . Syngeneic and allogeneic bone marrow engraftment after total body irradiation: dependence on dose, dose rate, and fractionation. Blood 1991; 77: 661–669.
Storb R, Raff RF, Appelbaum FR, Deeg HJ, Graham TC, Schuening FG et al. Fractionated versus single-dose total body irradiation at low and high dose rates to condition canine littermates for DLA-identical marrow grafts. Blood 1994; 83: 3384–3389.
Terenzi A, Aristei C, Aversa F, Pasqualucci L, Albi N, Velardi A et al. Comparison of immunosuppressive effects of single-dose and hyperfractionated total body irradiation. Transplant Proc 1994; 26: 3217.
Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med 1998; 339: 1186–1193.
Aristei C, Latini P, Terenzi A, Felicini R, Aversa F . Total body irradiation-based regimen in the conditioning of patients submitted to haploidentical stem cell transplantation. Radiother Oncol 2001; 58: 247–249.
Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S et al. Full haplotype- mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol 2005; 23: 3447–3454.
Mollee P, Gupta V, Song K, Reddy V, Califaretti N, Tsang R et al. Long-term outcome after intensive therapy with etoposide, melphalan, total body irradiation and autotransplant for acute myeloid leukemia. Bone Marrow Transplant 2004; 33: 1201–1208.
Chantry AD, Snowden JA, Craddock C, Peggs K, Roddie C, Craig JI et al. Long-term outcomes of myeloablation and autologous transplantation of relapsed acute myeloid leukemia in second remission: a British Society of Blood and Marrow Transplantation registry study. Biol Blood Marrow Transplant 2006; 12: 1310–1317.
Linker C, Damon L, Martin T, Blume K, Forman S, Snyder D et al. Autologous hematopoietic cell transplantation for high-risk ALL. Bone Marrow Transplant 2011; 33: 460–461.
Vellenga E, van Putten W, Ossenkoppele GJ, Verdonck LF, Theobald M, Cornelissen JJ et al. Autologous peripheral blood stem cell transplantation for acute myeloid leukemia. Blood 2011; 118: 6037–6042.
Huang J, Zou DH, Li ZJ, Fu MW, Xu Y, Zhao YZ et al. An auto-SCT-based total therapy resulted in encouraging outcomes in adolescents and young adults with acute lymphoblastic leukemia: report from a single center of China. Bone Marrow Transplant 2012; 47: 1087–1094.
Keating A, DaSilva G, Pérez WS, Gupta V, Cutler CS, Ballen KK et al. Autologous blood cell transplantation versus HLA-identical sibling transplantation for acute myeloid leukemia in first complete remission: a registry study from the Center for International Blood and Marrow Transplantation Research. Haematologica 2013; 98: 185–192.
Willemze AJ, Geskus RB, Noordijk EM, Kal HB, Egeler RM, Vossen JM . HLA-identical haematopoietic stem cell transplantation for acute leukaemia in children: less relapse with higher biologically effective dose of TBI. Bone Marrow Transplant 2007; 40: 319–327.
Van Kempen-Harteveld ML, Brand R, Kal HB, Verdonck LF, Hofman P, Schattenberg AV et al. Results of hematopoietic stem cell transplantation after treatment with different high-dose total-body irradiation regimens in five dutch centers. Int J Radiat Oncol Biol Phys 2008; 71: 1444–1454.
Girinsky T, Socie G, Ammarguellat H, Cosset J-M, Briot E, Bridier A et al. Consequences of two different doses to the lungs during a single dose of total body irradiation: results of a randomized study on 85 patients. Int J Radiat Oncol Biol Phys 1994; 30: 821–824.
Morgan TL, Falk PM, Kogut N, Shah KH, Tome M, Kagan AR . A comparison of single-dose and fractionated total-body irradiation on the development of pneumonitis following bone marrow transplantation. Int J Radiat Oncol Biol Phys 1996; 36: 61–66.
Wong JYC, Liu A, Schultheiss T, Popplewell L, Stein A, Rosenthal J et al. Targeted total marrow irradiation using three-dimensional image-guided tomographic intensity-modulated radiation therapy: an alternative to standard total body irradiation. Biol Blood Marrow Transplant 2006; 12: 306–315.
Wong JY, Rosenthal J, Liu A, Schultheiss T, Forman S, Somlo G . Image-guided total- marrow irradiation using helical tomotherapy in patients with multiple myeloma and acute leukemia undergoing hematopoietic cell transplantation. Int J Radiat Oncol Biol Phys 2009; 73: 273–279.
Corvò R, Zeverino M, Vagge S, Agostinelli S, Barra S, Taccini G et al. Helical tomotherapy targeting total bone marrow after total body irradiation for patients with relapsed acute leukemia undergoing an allogeneic stem cell transplant. Radiother Oncol 2011; 98: 382–386.
Acknowledgements
This work was partly supported by the ‘Quality Indicators for Radiotherapy’ Project, funded by the Italian Ministry of Health. The authors would like to thank Geraldine A Boyd for editing this paper. The authors would also like to thank, for having collected data, Paola Anselmo, Michele Benedetti, Massimo Berger, Francesco Borgia, Maria Samaritana Buzzaccarini, Silvia Durzu, Rita Felicini, Chiara Julita, Francesca Mascioni, Filippo Milano, Maddalena Giannini, Tindara Munafò, Dolores Tomasini, Stefania Varotto, Lisa Vicenzi, Elena Viganò, Elisabetta Vitali.
Italian TBI Working group
Cynthia Aristei: Università degli Studi e Azienda Ospedaliera - Struttura Complessa di Radioterapia Oncologica - Ospedale Santa Maria della Misericordia Perugia; Lorenzo Falcinelli: Struttura Complessa di Radioterapia Oncologica Azienda Ospedaliera Perugia; Gianni Gobbi, Carlo Raymondi: Servizio di Fisica Sanitaria; Azienda Ospedaliera, Ospedale Santa Maria della Misericordia, Perugia; Franco Aversa: Ematologia e Centro Trapianti Midollo Osseo, Università di Parma e Ospedale Maggiore, Parma; Antonella Santucci: Università degli Studi e Azienda Ospedaliera – Centro Trapianto Midollo Osseo - Santa Maria della Misericordia, Perugia
Enza Barbieri: Università di Bologna e Divisione di Radioterapia Ospedale S.Orsola Malpighi, Bologna; Andrea Ferri: Servizio di Fisica Sanitaria Ospedale S.Orsola Malpighi, Bologna; Giuseppe Bandini: Istituto di Ematologia Ospedale S.Orsola Malpighi, Bologna; Andrea Pession: Dipartimento Scienze Pediatriche, Ospedale S.Orsola Malpighi, Bologna
Stefano Maria Magrini: Istituto del Radio «O.Alberti» Università degli Studi e Spedali Civili, Brescia; Filippo Bertoni: UOC di Radioterapia Oncologica, Azienda Ospedaliero-Univeristaria Policlinico di Modena, Modena; Marco Galelli: Servizio di Fisica Sanitaria Spedali Civili, Brescia; Sandro Tonoli: Radioterapia Spedali Civili, Brescia; Michela Buglione di Monale e Bastia: Cattedra di Radioterapia, Università di Brescia
Renzo Corvò: Università degli Studi e Istituto Nazionale per la Ricerca sul Cancro, Genova; Franca Foppiano: Servizio di Fisica Sanitaria, ASL5, La Spezia; Salvina Barra: Divisione di Radioterapia Oncologica Istituto Nazionale per la Ricerca sul Cancro, Genova; Andrea Bacigalupo, Francesco Frassoni, Barbara Bruno: Centro Trapianto Midollo Osseo Ospedale San Martino, Genova; Giorgio Dini: Unità di Onco-Ematologia Pediatrica Ospedale Gaslini, Genova; Maura Faraci: Unità Trapianto Midollo Osseo Ospedale Gaslini, Genova
Gianstefano Gardani: U.O. di Radioterapia Oncologica Università di Milano «La Bicocca» e Ospedale S. Gerardo, Monza; Cornelio Uderzo: Ematologia Pediatrica Ospedale S. Gerardo, Monza; Andrea Crespi: Servizio di Fisica Sanitaria Ospedale S. Gerardo, Monza
Giovanni Scarzello: Radioterapia Azienda Ospedaliera, Padova; Roberto Zandonà: Servizio di Fisica Sanitaria Azienda Ospedaliera, Padova; Chiara Messina: Ematologia Azienda Ospedaliera, Padova
Vittorio Donato: Dipartimento di Radioterapia, Ospedale San Camillo-Forlanini, Roma; Lavinia Grapulin: Policlinico Umberto I - Radioterapia - Istituto di Radiologia Università «La Sapienza», Roma; Cinzia Di Felice, Elisabetta Di Castro: Policlinico Umberto I - Fisica Sanitaria - Istituto di Radiologia Università «La Sapienza», Roma; Anna Paola Iori: Policlinico Umberto I – Ematologia- Università «La Sapienza», Roma; Walter Barbieri: Policlinico Umberto I – Ematologia- Università «La Sapienza», Roma; William Arcese: Dipartimento di Ematologia, Unità di Trapianto, Università di Roma “Tor Vergata”, Roma
Michele Troiano, Salvatore Parisi: Divisione di Radioterapia Oncologica – IRCCS Casa Sollievo della Sofferenza, S.Giovanni Rotondo; Alberto Maiorana: Servizio di Fisica Sanitaria – IRCCS Casa Sollievo della Sofferenza, S.Giovanni Rotondo; Angelo Michele Carella: Divisione di Ematologia, IRCCS Azienda Ospedaliera Universitaria San Martino, Genova
Umberto Ricardi, Giuseppe Rossi, Andrea Riccardo Filippi: Radioterapia Ospedale San Giovanni Battista «Le Molinette», Torino; Riccardo Ragona: Servizio di Fisica Sanitaria Ospedale San Giovanni Battista «Le Molinette», Torino
Luigi Tomio: Radioterapia Oncologica, Ospedale Santa Chiara, Trento
Rosa Bianca Guglielmi, Cristina Baiocchi: Radioterapia Ospedale San Bortolo, Vicenza; Paolo Scalchi: Servizio di Fisica Sanitaria Ospedale San Bortolo, Vicenza; Roberto Raimondi: Ematologia Ospedale San Bortolo, Vicenza
Cristiana Vidali S.C.: Radioterapia Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Trieste; Natasha Maximova: Oncoematologia Pediatrica, Istituto per l’infanzia ed Ospedale specializzato pediatrico regionale - IRCCS “Burlo Garofolo”, Trieste
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Aristei, C., Santucci, A., Corvò, R. et al. In haematopoietic SCT for acute leukemia TBI impacts on relapse but not survival: results of a multicentre observational study. Bone Marrow Transplant 48, 908–914 (2013). https://doi.org/10.1038/bmt.2013.66
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.66
Keywords
This article is cited by
-
Light and shadows of a new technique: is photon total-skin irradiation using helical IMRT feasible, less complex and as toxic as the electrons one?
Radiation Oncology (2018)
-
Prognostic factors and outcomes for pediatric patients receiving an haploidentical relative allogeneic transplant using CD3/CD19-depleted grafts
Bone Marrow Transplantation (2016)
-
Hematopoietic SCT in Iranian children 1991–2012
Bone Marrow Transplantation (2015)