Abstract
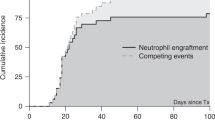
HLA-haploidentical hematopoietic SCT (HSCT) is an option for severe aplastic anemia (SAA) patients. Here, we evaluated the outcomes of 26 adult-SAA patients who received HLA-haploidentical HSCT in five transplant centers in southwestern China. Most of the patients in this study failed prior therapy and were transfused heavily before the transplantation. The patients received fludarabine+cyclophosphamide+antithymocyte globulin as conditioning regimens and then unmanipulated peripheral blood plus marrow transplantation. Micafungin, i.v. Ig and recombinant human TPO were used for post-grafting infection prevention and supportive care. Of 26 patients, 25 achieved engraftment at a median of 13 days (range, 11–19 days) after HSCT. One of 25 patients experienced graft rejection and did not achieve sustained engraftment after second HSCT. Therefore, the final engraftment rate was 92.3%. Three of 25 (12%) patients developed acute GVHD, 10 of 25 (40%) patients developed chronic GVHD (9 with limited whereas the other with extensive). The OS rate was 84.6% and the average follow-up time was 1313.2 (738–2005) days for surviving patients. This encouraging result suggests that HLA-haploidentical HSCT is an effective therapeutic option for adults with acquired SAA if an HLA-identical donor is not available.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Eapen M, Rademacher JL, Antin JH, Champlin RE, Carreras J, Fay J et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood 2011; 118: 2618–2621.
Klingebiel T, Cornish J, Labopin M, Locatelli F, Darbyshire P, Handgretinger R et al. Results and factors influencing outcome after fully haploidentical hematopoietic stem cell transplantation in children with very high-risk acute lymphoblastic leukemia: impact of center size: an analysis on behalf of the Acute Leukemia and Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Blood 2010; 115: 3437–3446.
Reisner Y, Hagin D, Martelli MF . Haploidentical hematopoietic transplantation: current status and future perspectives. Blood 2011; 118: 6006–6017.
Woodard P, Cunningham JM, Benaim E, Chen X, Hale G, Horwitz E et al. Effective donor lymphohematopoietic reconstitution after haploidentical CD34+-selected hematopoietic stem cell transplantation in children with refractory severe aplastic anemia. Bone Marrow Transplant 2004; 33: 411–418.
Tsutsumi Y, Tanaka J, Miura T, Saitoh S, Yamada M, Yamato H et al. Successful non-T-cell-depleted nonmyeloablative hematopoietic stem cell transplantation (NST) from an HLA-haploidentical 2-loci-mismatched sibling in a heavily transfused patient with severe aplastic anemia based on the fetomaternal microchimerism. Bone Marrow Transplant 2004; 34: 267–269.
Kremens B, Basu O, Grosse-Wilde H, Sauerwein W, Schaefer UW, Havers W . Transplantation of CD34-enriched peripheral stem cells from an HLA-haplotype mismatched donor to a patient with severe aplastic anemia. Bone Marrow Transplant 2001; 27: 111–113.
Wagner JL, Deeg HJ, Seidel K, Anasetti C, Doney K, Sanders J et al. Bone marrow transplantation for severe aplastic anemia from genotypically HLA-nonidentical relatives. An update of the Seattle experience. Transplantation 1996; 61: 54–61.
Xu LP, Liu KY, Liu DH, Han W, Chen H, Chen YH et al. A novel protocol for haploidentical hematopoietic SCT without in vitro T-cell depletion in the treatment of severe acquired aplastic anemia. Bone Marrow Transplant 2012; 47: 1507–1512.
Guinan EC . Diagnosis and management of aplastic anemia. Hematol Am Soc Hematol Educ Program 2011; 2011: 76–81.
Chen XH, Gao L, Zhang X, Gao L, Zhang C, Kong PY et al. HLA-haploidentical blood and bone marrow transplantation with anti-thymocyte globulin: long-term comparison with HLA-identical sibling transplantation. Blood Cells Mol Dis 2009; 43: 98–104.
Min CK, Kim DW, Lee JW, Han CW, Min WS, Kim CC . Hematopoietic stem cell transplantation for high-risk adult patients with severe aplastic anemia; reduction of graft failure by enhancing stem cell dose. Haematologica 2001; 86: 303–310.
Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol 1991; 28: 250–259.
Zaia JA . Cytomegalovirus infection. In: Blume KG, Forman SJ, Appelbaum FR, (eds). Hematopoitic Cell Transplantation 3rd edn Blackwell Publishing: Malden, MA, 2004, 701–726.
Passweg JR, Perez WS, Eapen M, Camitta BM, Gluckman E, Hinterberger W et al. Bone marrow transplants from mismatched related and unrelated donors for severe aplastic anemia. Bone Marrow Transplant 2006; 37: 641–649.
Stefan CO, Marcos L, Pedro C, Martin K, Sergio G, Elizabeth SJ et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation 2009; 88: 1019–1024.
Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood 2008; 112: 3574–3581.
Woodard P, Tong X, Richardson S, Srivastava DK, Horwitz EM, Benaim E et al. Etiology and outcome of graft failure in pediatric hematopoietic stem cell transplant recipients. J Pediatr Hematol Oncol 2003; 25: 955–959.
Kato S, Yabe H, Yasui M, Kawa K, Yoshida T, Watanabe A et al. Allogeneic hematopoietic transplantation of CD34þ selected cells from an HLA haplo-identical related donor. A long-term follow-up of 135 patients and a comparison of stem cell source between the bone marrow and the peripheral blood. Bone Marrow Transplant 2000; 26: 1281–1290.
Deeg HJ, Amylon ID, Harris RE, Collins R, Beatty PG, Feig S et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant 2001; 7: 208–215.
Carney DA, Westerman DA, Tam CS, Milner A, Prince HM, Kenealy M et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following fludarabine combination chemotherapy. Leukemia 2010; 24: 2056–2062.
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376: 1164–1174.
Chewning JH, Castro-Malaspina H, Jakubowski A, Kernan NA, Papadopoulos EB, Small TN et al. Fludarabine-based conditioning secures engraftment of second hematopoietic stem cell allografts (HSCT) in the treatment of initial graft failure. Biol Blood Marrow Transplant 2007; 13: 1313–1323.
Bacigalupo A, Locatelli F, Lanino E, Marsh J, Socié G, Maury S et al. Fludarabine, cyclophosphamide and anti-thymocyte globulin for alternative donor transplants in acquired severe aplastic anemia: a report from the EBMT-SAA Working Party. Bone Marrow Transplant 2005; 36: 947–950.
Basser RL, Rasko JE, Clarke K, Cebon J, Green MD, Hussein S et al. Thrombopoietic effects of pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) in patients with advanced cancer. Lancet 1996; 348: 1279–1281.
Liu DH, Huang XJ, Liu KY, Xu LP, Chen YH, Wang Y et al. Safety of recombinant human thrombopoietin in adults after related donor haploidentical haematopoietic stem cell transplantation a pilot study. Clin Drug Investig 2011; 31: 135–141.
Zhang C, Zhang X, Chen XH, Gao L, Gao L, Kong PY et al. Factors influencing engraftment in HLA-haploidentical/mismatch related transplantation with combined granulocyte-colony stimulating factor-mobilized peripheral blood and bone marrow for patients with leukemia. Transfus Apher Sci 2011; 44: 249–255.
Shaffer J, Villard J, Means TK, Alexander S, Dombkowski D, Dey BR et al. Regulatory T-cell recovery in recipients of haploidentical nonmyeloablative hematopoietic cell transplantation with a humanized anti-CD2 mAb, MEDI-507, with or without fludarabine. Exp Hematol 2007; 35: 1140–1152.
Li JM, Giver CR, Waller EK . Graft engineering using ex vivo methods to limit GVHD: fludarabine treatment generates superior GVL effects in allogeneic BMT. Exp Hematol 2006; 34: 895–904.
Palomo M, Diaz-Ricart M, Carbo C, Rovira M, Fernandez-Aviles F, Martine C et al. Endothelial dysfunction after hematopoietic stem cell transplantation: role of the conditioning regimen and the type of transplantation. Biol Blood Marrow Transplant 2010; 16: 985–993.
Im HJ, Koh KN, Choi ES, Jang S, Kwon SW, Park CJ et al. Excellent outcome of haploidentical hematopoietic stem cell transplantation in children and adolescents with acquired severe aplastic anemia. Biol Blood Marrow Transplant 2013; 19: 754–759.
Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O . Immunoglobulin prophylaxis in hematopoietic stem cell transplantation: systematic review and meta-analysis. J Clin Oncol 2008; 27: 770–781.
Huang XJ, Chen H, Han MZ, Zou P, Wu DP, Lai YR et al. Multicenter, randomized, open-label study comparing the efficacy and safety of micafungin versus itraconazole for prophylaxis of invasive fungal infections in patients undergoing hematopoietic stem cell transplant. Biol Blood Marrow Transplant 2012; 18: 1509–1516.
Acknowledgements
This research was supported in part by the Research Fund from the Natural Science Foundation of Chongqing (no. 2009BA5056), Clinical Foundation of TMMU and ‘1130’ Foundation of Xinqiao Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gao, L., Li, Y., Zhang, Y. et al. Long-term outcome of HLA-haploidentical hematopoietic SCT without in vitro T-cell depletion for adult severe aplastic anemia after modified conditioning and supportive therapy. Bone Marrow Transplant 49, 519–524 (2014). https://doi.org/10.1038/bmt.2013.224
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.224
Keywords
This article is cited by
-
Mesenchymal stromal cells as prophylaxis for graft-versus-host disease in haplo-identical hematopoietic stem cell transplantation recipients with severe aplastic anemia?—a systematic review and meta-analysis
Stem Cell Research & Therapy (2021)
-
Feasibility of reduced-dose posttransplant cyclophosphamide and cotransplantation of peripheral blood stem cells and umbilical cord-derived mesenchymal stem cells for SAA
Scientific Reports (2021)
-
Comparison of efficacy and health-related quality of life of first-line haploidentical hematopoietic stem cell transplantation with unrelated cord blood infusion and first-line immunosuppressive therapy for acquired severe aplastic anemia
Leukemia (2020)
-
Haploidentical hematopoietic stem cell transplantation in aplastic anemia: a systematic review and meta-analysis of clinical outcome on behalf of the severe aplastic anemia working party of the European group for blood and marrow transplantation (SAAWP of EBMT)
Bone Marrow Transplantation (2020)
-
Haploidentical transplantation and posttransplant cyclophosphamide for treating aplastic anemia patients: a report from the EBMT Severe Aplastic Anemia Working Party
Bone Marrow Transplantation (2020)