Abstract
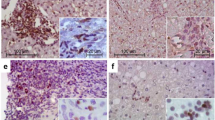
The contribution of Th17 cells to chronic GVHD (cGVHD) has been demonstrated in cGVHD mouse models. However, their contribution to human liver cGVHD remains unclear. We evaluated Th17 cells in biopsies from a cohort of 17 patients with liver cGVHD. We observed a significant increase in Th17 cells in the liver of patients with cGVHD, as demonstrated by an increase in CCR6+, CD161+ and RORγt+ T cells (P=0.03, P=0.0001 and P=0.03, respectively). We also assessed the presence of Th1 and regulatory (Treg) T cells: the numbers of Th1 and Treg cells were very low, with no difference between the two groups (P=0.88 and P=0.12, respectively). Furthermore, Th17/Th1 and Th17/Treg ratios were significantly increased in the liver of patients with liver cGVHD (P=0.005 and P=0.002, respectively). This study provides evidence for an infiltration by Th17 cells in the liver of patients with cGVHD and an increased Th17/Treg ratio, suggesting a defect in the regulatory mechanism driven by Treg cells or an inappropriate activation of effectors cells, especially Th17 cells, or both mechanisms, in human liver cGVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ferrara JL, Levine JE, Reddy P, Holler E . Graft-versus-host disease. Lancet 2009; 373: 1550–1561.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Murata M, Fujimoto M, Matsushita T, Hamaguchi Y, Hasegawa M, Takehara K et al. Clinical association of serum interleukin-17 levels in systemic sclerosis: is systemic sclerosis a Th17 disease? J Dermatol Sci 2008; 50: 240–242.
Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S . Identification of IL-18 and Th17 cells in salivary glands of patients with Sjogren's syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol 2008; 181: 2898–2906.
Espinosa A, Dardalhon V, Brauner S, Ambrosi A, Higgs R, Quintana FJ et al. Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23-Th17 pathway. J Exp Med 2009; 206: 1661–1671.
Chen X, Das R, Komorowski R, van Snick J, Uyttenhove C, Drobyski WR . Interleukin 17 is not required for autoimmune-mediated pathologic damage during chronic graft-versus-host disease. Biol Blood Marrow Transplant 2010; 16: 123–128.
Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK . Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med 2006; 203: 2785–2791.
Chen X, Vodanovic-Jankovic S, Johnson B, Keller M, Komorowski R, Drobyski WR . Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood 2007; 110: 3804–3813.
Hill GR, Olver SD, Kuns RD, Varelias A, Raffelt NC, Don AL et al. Stem cell mobilization with G-CSF induces type 17 differentiation and promotes scleroderma. Blood 2010; 116: 819–828.
Nishimori H, Maeda Y, Teshima T, Sugiyama H, Kobayashi K, Yamasuji Y et al. Synthetic retinoid Am80 ameliorates chronic graft-versus-host disease by down-regulating Th1 and Th17. Blood 2012; 119: 285–295.
Ritchie D, Seconi J, Wood C, Walton J, Watt V . Prospective monitoring of tumor necrosis factor alpha and interferon gamma to predict the onset of acute and chronic graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2005; 11: 706–712.
Dander E, Balduzzi A, Zappa G, Lucchini G, Perseghin P, Andre V et al. Interleukin-17-producing T-helper cells as new potential player mediating graft-versus-host disease in patients undergoing allogeneic stem-cell transplantation. Transplantation 2009; 88: 1261–1272.
Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant 2006; 12: 31–47.
Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B et al. Phenotypic and functional features of human Th17 cells. J Exp Med 2007; 204: 1849–1861.
Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 2007; 8: 639–646.
Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med 2009; 206: 525–534.
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006; 126: 1121–1133.
Grogan BM, Tabellini L, Storer B, Bumgarner TE, Astigarraga CC, Flowers ME et al. Activation and expansion of CD8(+) T effector cells in patients with chronic graft-versus-host disease. Biol Blood Marrow Transplant 2011; 17: 1121–1132.
Yamashita K, Horwitz ME, Kwatemaa A, Nomicos E, Castro K, Sokolic R et al. Unique abnormalities of CD4(+) and CD8(+) central memory cells associated with chronic graft-versus-host disease improve after extracorporeal photopheresis. Biol Blood Marrow Transplant 2006; 12: 22–30.
D' Asaro M, Salerno A, Dieli F, Caccamo N . Analysis of memory and effector CD8+ T cell subsets in chronic graft-versus-host disease. Int J Immunopathol Pharmacol 2009; 22: 195–205.
Croudace JE, Inman CF, Abbotts BE, Nagra S, Nunnick J, Mahendra P et al. Chemokine-mediated tissue recruitment of CXCR3+ CD4+ T-cells plays a major role in the pathogenesis of chronic graft versus host disease. Blood 2012; 120: 4246–4255.
De Wit D, Van Mechelen M, Zanin C, Doutrelepont JM, Velu T, Gerard C et al. Preferential activation of Th2 cells in chronic graft-versus-host reaction. J Immunol 1993; 150: 361–366.
Ochs LA, Blazar BR, Roy J, Rest EB, Weisdorf DJ . Cytokine expression in human cutaneous chronic graft-versus-host disease. Bone Marrow Transplant 1996; 17: 1085–1092.
Faber LM, van Luxemburg-Heijs SA, Veenhof WF, Willemze R, Falkenburg JH . Generation of CD4+ cytotoxic T-lymphocyte clones from a patient with severe graft-versus-host disease after allogeneic bone marrow transplantation: implications for graft-versus-leukemia reactivity. Blood 1995; 86: 2821–2828.
Korholz D, Kunst D, Hempel L, Sohngen D, Heyll A, Bonig H et al. Decreased interleukin 10 and increased interferon-gamma production in patients with chronic graft-versus-host disease after allogeneic bone marrow transplantation. Bone Marrow Transplant 1997; 19: 691–695.
Nistala K, Adams S, Cambrook H, Ursu S, Olivito B, de Jager W et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci USA 2010; 107: 14751–14756.
Lee YK, Mukasa R, Hatton RD, Weaver CT . Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol 2009; 21: 274–280.
Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood 2005; 106: 2903–2911.
Li Q, Zhai Z, Xu X, Shen Y, Zhang A, Sun Z et al. Decrease of CD4(+)CD25(+) regulatory T cells and TGF-beta at early immune reconstitution is associated to the onset and severity of graft-versus-host disease following allogeneic haematogenesis stem cell transplantation. Leuk Res 2010; 34: 1158–1168.
Ratajczak P, Janin A, Peffault de Latour R, Leboeuf C, Desveaux A, Keyvanfar K et al. Th17/Treg ratio in human graft-versus-host disease. Blood 2010; 116: 1165–1171.
Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 2012; 366: 1190–1199.
Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 2012; 366: 1181–1189.
Acknowledgements
The authors acknowledge the technical and logistical support of S Blandin, C Deleine, S Leclercq and V Dehame. We also thank the nursing staff for providing excellent care for our patients, and the following physicians: N Blin, A Clavert, V Dubruille, T Gastinne, JL Harousseau, S Le Gouill, B Mahe, F Mechinaud and F Rialland for their dedicated patient care. FM and EB were supported by educational grants from the ‘Association for Training, Education and Research in Hematology, Immunology and Transplantation’ (ATERHIT). This work was supported by funding as part of the CESTI project (Nantes, France). We also thank the ‘Région Pays de Loire’, the ‘Association pour la Recherche sur le Cancer (ARC; grant #3175 to MM and BG)’, the ‘Fondation de France’, the ‘Fondation contre la Leucémie’, the ‘Agence de Biomédecine’, the ‘Association CentpourSang la Vie’, the ‘Association Laurette Fuguain’, the IRGHET and the ‘Ligue Contre le Cancer’ (Comités Grand-Ouest) for their generous and continuous support of our clinical and basic research work. Our transplant programs are supported by several grants from the French national cancer institute (PHRC, INCa to MM). The authors acknowledge the continuous support of the cell banking facility (‘tumorotheque’) of the CHU de Nantes.
Author contributions
All authors listed in the manuscript have contributed substantially to this work: conception and design: Florent Malard, Mohamad Mohty, Béatrice Gaugler, Céline Bossard; financial support: Mohamad Mohty, Marc Grégoire; administrative and logistical support: Jean-François Mosnier, Mohamad Mohty, Béatrice Gaugler, Marc Grégoire; provision of study materials and patients care: Eolia Brissot, Patrice Chevallier, Thierry Guillaume, Jacques Delaunay, Philippe Moreau, Mohamad Mohty; experimental work: Florent Malard, Céline Bossard; collection and assembly of clinical data: Florent Malard, Eolia Brissot, Mohamad Mohty; data analysis and interpretation: Florent Malard, Céline Bossard, Mohamad Mohty, Béatrice Gaugler; manuscript writing: Florent Malard, Mohamad Mohty, Céline Bossard, Béatrice Gaugler; final approval of manuscript: all co-authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Malard, F., Bossard, C., Brissot, E. et al. Increased Th17/Treg ratio in chronic liver GVHD. Bone Marrow Transplant 49, 539–544 (2014). https://doi.org/10.1038/bmt.2013.215
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.215
Keywords
This article is cited by
-
Regulatory T Cell Plasticity and Stability and Autoimmune Diseases
Clinical Reviews in Allergy & Immunology (2020)
-
Diverse Activity of IL-17+ Cells in Chronic Skin and Mucosa Graft-Versus-Host Disease
Archivum Immunologiae et Therapiae Experimentalis (2019)
-
A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing cGVHD
Journal of Hematology & Oncology (2018)
-
Risk Factors and Outcomes of Invasive Fungal Infections in Allogeneic Hematopoietic Cell Transplant Recipients
Mycopathologia (2017)
-
GRIM19 ameliorates acute graft-versus-host disease (GVHD) by modulating Th17 and Treg cell balance through down-regulation of STAT3 and NF-AT activation
Journal of Translational Medicine (2016)