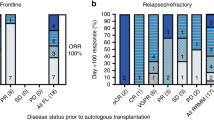
Abstract
In patients with relapsed ALL, minimal residual disease (MRD) identified prior to allogeneic hematopoietic cell transplantation (HCT) is a strong predictor of relapse. We report our experience using a combination of reduced-dosing clofarabine, CY and etoposide as a ‘bridge’ to HCT in eight patients with high risk or relapsed ALL and pre-HCT MRD. All patients had detectable MRD (>0.01%, flow cytometry) at the start of therapy with all eight achieving MRD reduction following one cycle. The regimen was well tolerated with seven grade 3/4 toxicities occurring among four of the eight patients. Five patients (62.5%) are alive, one died from relapse (12.5%) and two from transplant-related mortality (25%). The combination of reduced-dose clofarabine, CY and etoposide as bridging therapy appears to be well tolerated in patients with relapsed ALL and is effective in reducing pre-HCT MRD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hunger SP, Loh ML, Whitlock JA, Winick NJ, Carroll WL, Devidas M et al. Children’s Oncology Group’s 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer 2012; 60: 957–963.
Raetz EA, Bhatla T . Where do we stand in the treatment of relapsed acute lymphoblastic leukemia? Hematol Am Soc Hematol Educ Program 2012; 2012: 129–136.
Ruggeri A, Michel G, Dalle JH, Caniglia M, Locatelli F, Campos A et al. Impact of pretransplant minimal residual disease after cord blood transplantation for childhood acute lymphoblastic leukemia in remission: an Eurocord, PDWP-EBMT analysis. Leukemia 2012; 26: 2455–2461.
Bachanova V, Burke MJ, Yohe S, Cao Q, Sandhu K, Singleton TP et al. Unrelated cord blood transplantation in adult and pediatric acute lymphoblastic leukemia: effect of minimal residual disease on relapse and survival. Biol Blood Marrow Transplant 2012; 18: 963–968.
Pulsipher MA, Bader P, Klingebiel T, Cooper LJ . Allogeneic transplantation for pediatric acute lymphoblastic leukemia: the emerging role of peritransplantation minimal residual disease/chimerism monitoring and novel chemotherapeutic, molecular, and immune approaches aimed at preventing relapse. Biol Blood Marrow Transplant 2009; 15: 62–71.
Elorza I, Palacio C, Dapena JL, Gallur L, de Toledo JS, de Heredia CD . Relationship between minimal residual disease measured by multiparametric flow cytometry prior to allogeneic hematopoietic stem cell transplantation and outcome in children with acute lymphoblastic leukemia. Haematologica 2010; 95: 936–941.
Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol 2009; 27: 377–384.
Foster JH, Hawkins DS, Loken MR, Wells DA, Thomson B . Minimal residual disease detected prior to hematopoietic cell transplantation. Pediatr Blood Cancer 2011; 57: 163–165.
Burke MJ, Lindgen B, Verneris MR . Treatment of relapsed acute lymphoblastic leukemia: approaches used by pediatric oncologists and bone marrow transplant physicians. Pediatr Blood Cancer 2012; 58: 840–845.
Jeha S, Gandhi V, Chan KW, McDonald L, Ramirez I, Madden R et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood 2004; 103: 784–789.
Hijiya N, Thomson B, Isakoff MS, Silverman LB, Steinherz PG, Borowitz MJ et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood 2011; 118: 6043–6049.
Locatelli F, Testi AM, Bernardo ME, Rizzari C, Bertaina A, Merli P et al. Clofarabine, cyclophosphamide and etoposide as single-course re-induction therapy for children with refractory/multiple relapsed acute lymphoblastic leukaemia. Br J Haematol 2009; 147: 371–378.
Hijiya N, Gaynon P, Barry E, Silverman LB, Thomson B, Chu R et al. A multi-center phase I study of clofarabine, etoposide and cyclophosphamide in combination in pediatric patients with refractory or relapsed acute leukemia. Leukemia 2009; 23: 2259–2264.
Leung W, Pui CH, Coustan-Smith E, Yang J, Pei D, Gan K et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood 2012; 120: 468–472.
Wayne AS, Radich JP . Pretransplant MRD: the light is yellow, not red. Blood 2012; 120: 244–246.
Acknowledgements
Children’s Cancer Research Fund (MJB, MRV) and the University of Minnesota Pediatric Leukemia Program supported this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gossai, N., Verneris, M., Karras, N. et al. A clofarabine-based bridging regimen in patients with relapsed ALL and persistent minimal residual disease (MRD). Bone Marrow Transplant 49, 440–442 (2014). https://doi.org/10.1038/bmt.2013.195
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.195
Keywords
This article is cited by
-
Combination of clofarabine, etoposide, and cyclophosphamide in adult relapsed/refractory acute lymphoblastic leukemia: a phase 1/2 dose-escalation study by the Japan Adult Leukemia Study Group
International Journal of Hematology (2021)
-
Refractory acute lymphoblastic leukemia in Chinese children: bridging to stem cell transplantation with clofarabine, cyclophosphamide and etoposide
Annals of Hematology (2016)
-
Clofarabine/cyclophosphamide/etoposide
Reactions Weekly (2015)