Abstract
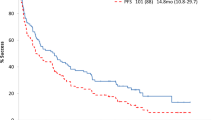
In Ig light chain (AL) amyloidosis, cardiac involvement is associated with worse prognosis and increased treatment-related complications. In this retrospective cohort study, we assessed survival, hematologic and cardiac responses to high-dose melphalan and auto-SCT (HDM/SCT) in patients with AL amyloidosis and cardiac involvement, stratified by cardiac biomarkers brain natriuretic peptide and Troponin I, analogous to the Mayo cardiac staging. Forty-seven patients underwent HDM/SCT based upon functional measures; six patients had modified cardiac stage I disease, seventeen had modified cardiac stage II disease and twenty-four had modified cardiac stage III disease. Treatment-related mortality was 4% for all patients and 8% for patients with stage III disease. Three-year survival was 88% and EFS was 47%; these did not differ by stage. By intention-to-treat analysis, 27% of patients achieved a hematologic complete response and 32% a very good partial response, of whom 70 and 45%, respectively, have not required additional therapy at 36 months. Cardiac response was achieved in 53% of patients. We conclude that with appropriate patient selection and a risk-adapted treatment approach, HDM/SCT is safe and effective in patients with AL amyloidosis and cardiac involvement.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM 1998; 91: 141–157.
Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol 2004; 22: 3751–3757.
Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM et al. Prognostication of survival using cardiac troponins and N-terminal pro-brain natriuretic peptide in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood 2004; 104: 1881–1887.
Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol 2012; 30: 989–995.
Palladini G, Barassi A, Klersy C, Pacciolla R, Milani P, Sarais G et al. The combination of high-sensitivity cardiac troponin T (hs-cTnT) at presentation and changes in N-terminal natriuretic peptide type B (NT-proBNP) after chemotherapy best predicts survival in AL amyloidosis. Blood 2010; 116: 3426–3430.
Wechalekar AD, Schonland SO, Kastritis E, Gillmore JD, Dimopoulos MA, Lane T et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood 2013; 121: 3420–3427.
Madan S, Kumar SK, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK et al. High-dose melphalan and peripheral blood stem cell transplantation for light-chain amyloidosis with cardiac involvement. Blood 2012; 119: 1117–1122.
Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol 2005; 79: 319–328.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA et al. Recommendations for chamber quantification. Eur J Echocardiogr 2006; 7: 79–108.
Masson S, Vago T, Baldi G, Salio M, De Angelis N, Nicolis E et al. Comparative measurement of N-terminal pro-brain natriuretic peptide and brain natriuretic peptide in ambulatory patients with heart failure. Clin Chem Lab Med 2002; 40: 761–763.
McCullough PA, Omland T, Maisel AS . B-type natriuretic peptides: a diagnostic breakthrough for clinicians. Rev Cardiovasc Med 2003; 4: 72–80.
Skinner M, Sanchorawala V, Seldin DC, Dember LM, Falk RH, Berk JL et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med 2004; 140: 85–93.
Cibeira MT, Sanchorawala V, Seldin DC, Quillen K, Berk JL, Dember LM et al. Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood 2011; 118: 4346–4352.
Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol 2012; 30: 4541–4549.
Gertz MA, Lacy MQ, Dispenzieri A, Kumar SK, Dingli D, Leung N et al. Refinement in patient selection to reduce treatment-related mortality from autologous stem cell transplantation in amyloidosis. Bone Marrow Transplant 2013; 48: 557–561.
Girnius S, Seldin DC, Cibeira MT, Sanchorawala V . New hematologic response criteria predict survival in patients with immunoglobulin light chain amyloidosis treated with high-dose melphalan and autologous stem-cell transplantation. J Clin Oncol 2013; 31: 2749–2750.
Sanchorawala V, Seldin DC, Magnani B, Skinner M, Wright DG . Serum free light-chain responses after high-dose intravenous melphalan and autologous stem cell transplantation for AL (primary) amyloidosis. Bone Marrow Transplant 2005; 36: 597–600.
Jaccard A, Moreau P, Leblond V, Leleu X, Benboubker L, Hermine O et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med 2007; 357: 1083–1093.
Acknowledgements
We gratefully acknowledge our colleagues in the Clinical Trials Office, specifically Anthony Shelton, RN, Dina Brauneis, NP, and Kathleen T Finn, NP, and the staff of the Solomont Center for Cancer and Blood Disorders at Boston Medical Center who assisted with the multi-disciplinary evaluation and treatment of the patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Girnius, S., Seldin, D., Meier-Ewert, H. et al. Safety and efficacy of high-dose melphalan and auto-SCT in patients with AL amyloidosis and cardiac involvement. Bone Marrow Transplant 49, 434–439 (2014). https://doi.org/10.1038/bmt.2013.192
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.192
Keywords
This article is cited by
-
Comprehensive Review of AL amyloidosis: some practical recommendations
Blood Cancer Journal (2021)
-
Autologous stem cell transplantation in light-chain amyloidosis
memo - Magazine of European Medical Oncology (2021)
-
High-dose melphalan and autologous peripheral blood stem cell transplantation in patients with AL amyloidosis and cardiac defibrillators
Bone Marrow Transplantation (2019)
-
High-dose melphalan and stem cell transplantation in systemic AL amyloidosis in the era of novel anti-plasma cell therapy: a comprehensive review
Bone Marrow Transplantation (2019)
-
High-dose melphalan and stem cell transplantation in AL amyloidosis with elevated cardiac biomarkers
Bone Marrow Transplantation (2018)