Abstract
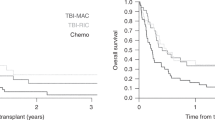
Previous data suggested that allo-SCT might be an effective therapy in the setting of chemo-refractory/relapsed diseases because of the potent long-term immune-mediated tumor control. This retrospective study aimed to analyze the outcome of adult patients who received allo-SCT in a chemo-refractory/relapsed status. The series included 840 patients with active or progressive disease at the time of transplant. Median age was 50 years. With a median follow-up of 40 months, 3-year OS, disease-free survival (DFS), and non-relapse mortality rates were 29±2, 23±2, and 30±2%, respectively. At the last follow-up, 252 patients (30%) were still alive (of whom 201 were in CR (24%). In a Cox multivariate analysis, the use of a reduced-intensity conditioning (RIC) before allo-SCT and use of an HLA-identical sibling donor remained independently associated with a better OS (hazard ratio (HR)=0.82; 95% confidence interval (CI), 0.69–0.98, P=0.03; and HR=0.79; 95% CI, 0.66–0.93, P=0.006, respectively). Also, a diagnosis of myelodysplastic syndrome/myeloproliferative disorder, Hodgkin lymphoma and non-Hodgkin lymphoma compared with acute leukemia had a favorable impact on OS (HR=0.55; 95% CI, 0.45–0.68, P<0.0001; HR=0.49; 95% CI, 0.31–0.75, P=0.001; and HR=0.47; 95% CI, 0.35–0.63, P<0.0001, respectively). In conclusion, this study suggests that allo-SCT may be of benefit in some subgroups of patients with active or progressive hematological malignancies at the time of allo-SCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Copelan EA . Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354: 1813–1826.
Luznik L, Fuchs EJ . Donor lymphocyte infusions to treat hematologic malignancies in relapse after allogeneic blood or marrow transplantation. Cancer Control 2012; 9: 123–137.
Gyurkocza B, Rezvani A, Storb RF . Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol 2010; 3: 285–299.
Passweg JR, Halter J, Bucher C, Gerull S, Heim D, Rovo A et al. Hematopoietic stem cell transplantation: a review and recommendations for follow-up care for the general practitioner. Swiss Med Wkly 2012; 142: w13696.
Passweg JR, Baldomero H, Gratwohl A, Bregni M, Cesaro S, Dreger P et al. The EBMT activity survey: 1990–2010. Bone Marrow Transplant 2012; 47: 906–923.
Mohty M, Labopin M, Balère ML, Socie G, Milpied N, Tabrizi R et al. Antithymocyte globulins and chronic graft-vs-host disease after myeloablative allogeneic stem cell transplantation from HLA-matched unrelated donors: a report from the Société Francaise de Greffe de Moelle et de Thérapie Cellulaire. Leukemia 2010; 24: 1867–1874.
Deeg HJ, Sandmaier BM . Who is fit for allogeneic transplantation? Blood 2010; 116: 4762–4770.
Ljungman P, Bregni M, Brune M, Cornelissen J, de Witte T, Dini G et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant 2010; 45: 219–234.
Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity qconditioning for allogeneic stem cell transplantation. Blood 2006; 108: 1092–1099.
Duval M, Klein JP, He Wensheng, Cahn JY, Cairo M, Camitta BM et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol 2010; 28: 3730–3738.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Fine JP, Gray RJ . A proportional hazards model for subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
Yanada M, Naoe T, Iida H, Sakamaki H, Sakura T, Kanamori H et al. Myeloablative allogeneic hematopoietic stem cell transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia in adults: significant roles of total body irradiation and chronic graft-versus-host disease. Bone Marrow Transplant 2005; 36: 867–872.
Gratwohl A, Brand R, Apperley J, Crawley C, Ruutu T, Corradini P et al. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: transplant activity, long-term data and current results. An analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Haematologica 2006; 91: 513–521.
Mohty M, Labopin M, Tabrizzi R, Theorin N, Fauser A, Rambaldi A et al. Reduced-intensity conditioning allogeneic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Haematologica 2008; 93: 303–306.
Terwey TH, Massenkeil G, Tamm I, Hemmati PG, Neuburger S, Martus P et al. Allogeneic SCT in refractory or relapsed adult ALL is effective without prior reinduction chemotherapy. Bone Marrow Transplant 2008; 42: 791–798.
Nishiwaki S, Inamoto Y, Sakamaki H, Kurokawa M, Iida H, Ogawa H et al. Allogeneic stem cell transplantation for adult Philadelphia chromosome-negative acute lymphocytic leukemia: comparable survival rates but different risk factors between related and unrelated transplantation in first complete remission. Blood 2010; 116: 4368–4375.
Craddock C, Labopin M, Pillai S, Finke J, Bunjes D, Greinix H et al. Fcators predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia 2011; 25: 808–813.
Bashir Q, Khan H, Orlowski RZ, Amjad AL, Shah N, Parmar S et al. Predictors of prolongad survival alter allogeneic hematopoietic stem cell transplantation for multiple myeloma. Am J Hematol 2012; 87: 272–276.
Bertz H, Illerhaus G, Veelken H, Finke J . Allogeneic hematopoietic stem-cell transplantation for patients with relapsed or refractory lymphomas: comparison of high-dose conventional conditioning versus fludarabine-based reduced-intensity regimens. Ann Oncol 2002; 13: 135–139.
Anderlini P, Saliba R, Acholonu S, Giralt SA, Andersson B, Ueno NT et al. Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin’s lymphoma: the updated M.D. Anderson Cancer Center experience. Haematologica 2008; 93: 257–264.
Hari P, Carreras J, Zhang MJ, Gale RP, Bolwell BJ, Bredeson CN et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant 2008; 14: 236–245.
Van Kampen RJ, Canals C, Schouten HC, Nagler A, Thomson KJ, Vernant JP et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin’s lymphoma relapsing aftera n autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol 2011; 29: 1342–1348.
Le Gouill S, Kroger N, Dhedin N, Nagler A, Bouabdallah K, Yakoub-Agha I et al. Reduced-intensity conditioning allogeneic stem cell transplantation for relapsed/refractory mantle cell lymphoma: a multicenter experience. Ann Oncol 2012; 23: 2695–2703.
Detrait MY, Chevallier P, Sobh M, Guillaume T, Thomas X, Morisset S et al. Outcome of high-risk and refractory AML/MDS patients receiving a FLAMSA sequential chemotherapy regimen followed by reduced-intensity conditioning (RIC) and allogeneic hematopoietic stem cell transplantation (allo-HSCT). Blood (ASH Annual Meeting Abstracts) 2011; 118 Abstract 1957.
Shimoni A, Nagler A . Optimizing the conditioning regimen for allogeneic stem-cell transplantation in acute myeloid leukemia; dose intensity is still need. Best Pract Res Clin Haematol 2011; 24: 369–379.
Mohty M, Chevallier P . Azacitidine after allo-SCT: the good without the bad? Blood 2012; 119: 3199–3200.
Chevallier P, Delaunay J, Turlure P, Pigneux A, Hunault M, Garand R et al. Long term disease free survival after the MIDAM (Mylotarg, intermediaite dose Ara-C, Mitoxantrone) regimen in patients with CD33+ primary resistant or relapsed acute myeloid leukemia. J Clin Oncol 2008; 26: 5192–5197.
Takahashi K, Kantarjian H, Pemmaraju N, Andreeff M, Borthakur G, Faderl S et al. Salvage therapy using FLT3 inhibitors may improve long-term outcome of relapsed or refractory AML in patients with FLT3-ITD. Br J Haematol 2013; 161: 659–666.
Acknowledgements
We thank all the data managers of each centre for data management and collection.
Author Contributions
PC conceived and designed the study, analyzed data, recruited patients, provided clinical care, performed bibliographic search and wrote the manuscript. MM conceived and designed the study, recruited patients, provided clinical care, analyzed data, performed bibliographic search and helped writing the manuscript. ML performed statistical analyses. NM, KB, GS, IY-A, MM, CEB, SM, YB, J-OB, DB, NM, GG, ED recruited patients, provided clinical care and commented on the manuscript. NR performed central data management and collection. All the authors approved the manuscript for publication purposes.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This study was presented as an oral session during the 37th Annual Meeting of the European Group for Blood and Marrow Transplantation (Bone Marrow Transplant 2011; Vol 46 (Supp1): S47 (abstract 0290)).
Rights and permissions
About this article
Cite this article
Chevallier, P., Labopin, M., Milpied, N. et al. Outcomes of adults with active or progressive hematological malignancies at the time of allo-SCT: a survey from the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). Bone Marrow Transplant 49, 361–365 (2014). https://doi.org/10.1038/bmt.2013.186
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.186
Keywords
This article is cited by
-
Cladribine-based debulking chemotherapy sequential with reduced intensity regimen and post-transplantation maintenance for refractory myeloid leukemia: a prospective phase II clinical trial
Bone Marrow Transplantation (2022)
-
Sequential allogeneic hematopoietic stem cell transplantation for active refractory/relapsed myeloid malignancies: results of a reduced-intensity conditioning preceded by clofarabine and cytosine arabinoside, a retrospective study on behalf of the SFGM-TC
Annals of Hematology (2020)
-
Outcomes of strategic alternative donor selection or suspending donor search based on Japan Marrow Donor Program coordination status
International Journal of Hematology (2018)
-
Long-term outcomes of allogeneic hematopoietic cell transplantation with intensified myeloablative conditioning for refractory myeloid malignancy
Bone Marrow Transplantation (2016)
-
Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study
Bone Marrow Transplantation (2016)