Abstract
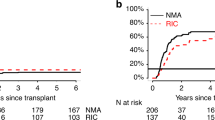
Reduced-intensity conditioning (RIC) allogeneic hematopoietic cell transplantation (allo-HCT) can cure patients with AML in CR. However, relapse after RIC allo-HCT may indicate heterogeneity in the stringency of CR. Strict definition of CR requires no evidence of leukemia by both morphologic and flow cytometric criteria. We re-evaluated 85 AML patients receiving RIC allo-HCT in CR to test if a strict definition of CR had direct implications for the outcome. These patients had leukemia immunophenotype documented at diagnosis and analyzed at allo-HCT. Eight (9.4%) had persistent leukemia by flow cytometric criteria at allo-HCT. The patients with immunophenotypic persistent leukemia had a significantly increased relapse (hazard ratio (HR): 3.7; 95% confidence interval (CI): 1.3–10.3, P=0.01) and decreased survival (HR: 2.9; 95% CI: 1.3–6.4, P<0.01) versus 77 patients in CR by both morphology and flow cytometry. However, the pre-allo-HCT bone marrow (BM) blast count (that is, 0–4%) was not significantly associated with risks of relapse or survival. These data indicate the presence of leukemic cells, but not the BM blast count affects survival. A strict morphologic and clinical lab flow cytometric definition of CR predicts outcomes after RIC allo-HCT, and therefore is critical to achieve at transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gupta V, Tallman MS, Weisdorf DJ . Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood 2011; 117: 2307–2318.
Oran B, Wagner JE, DeFor TE, Weisdorf DJ, Brunstein CG . Effect of conditioning regimen intensity on acute myeloid leukemia outcomes after umbilical cord blood transplantation. Biol Blood Marrow Transplant 2011; 17: 1327–1334.
Ringden O, Labopin M, Gluckman E, Reiffers J, Vernant JP, Jouet JP et al. Graft-versus-leukemia effect in allogeneic marrow transplant recipients with acute leukemia is maintained using cyclosporin A combined with methotrexate as prophylaxis. Bone Marrow Transplant 1996; 18: 921–929.
Burnett AK, Wheatley K, Goldstone AH, Stevens RF, Hann IM, Rees JHK et al. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: Results of the UK MRC AML 10 trial. Br J Haematol 2002; 118: 385–400.
Cornelissen JJ, van Putten WLJ, Verdonck LF, Theobald M, Jacky E, Daenen SMG et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood 2007; 109: 3658–3666.
Sandmaier BM, Maloney DG, McSweeney P, Niederwieser D, Shizuru J, Chauncey J et al. Nonmyeloablative conditioning for stem cell allografts with low-dose TBI: an update of toxicity and outcome. Exp Hematol 2000; 28: 60–60.
Majhail NS, Brunstein CG, Shanley R, Sandhu K, McClune B, Oran B et al. Reduced-intensity hematopoietic cell transplantation in older patients with AML/MDS: umbilical cord blood is a feasible option for patients without HLA-matched sibling donors. Bone Marrow Transplant 2012; 47: 494–498.
Mohty M, de Lavallade H, El-Cheikh J, Ladaique P, Faucher C, Furst S et al. Reduced intensity conditioning allogeneic stem cell transplantation for patients with acute myeloid leukemia: long term results of a 'donor' versus 'no donor' comparison. Leukemia 2009; 23: 194–196.
Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol 2005; 23: 1993–2003.
Goldman FD, Rumelhart SL, DeAlacron P, Holida MD, Lee NF, Miller J et al. Poor outcome in children with refractory/relapsed leukemia undergoing bone marrow transplantation with mismatched family member donors. Bone Marrow Transplant 2000; 25: 943–948.
Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol 2010; 28: 3730–3738.
Michallet M, Thomas X, Vernant JP, Kuentz M, Socie G, Esperou-Bourdeau H et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Societe Francaise de Greffe de Moelle (SFGM). Bone Marrow Transplant 2000; 26: 1157–1163.
Nemecek ER, Gooley TA, Woolfrey AE, Carpenter PA, Matthews DC, Sanders JE . Outcome of allogeneic bone marrow transplantation for children with advanced acute myeloid leukemia. Bone Marrow Transplant 2004; 34: 799–806.
Kebriaei P, Kline J, Stock W, Kasza K, Le Beau MM, Larson RA et al. Impact of disease burden at time of allogeneic stem cell transplantation in adults with acute myeloid leukemia and myelodysplastic syndromes. Bone Marrow Transplant 2005; 35: 965–970.
Blum W, Bolwell BJ, Phillips G, Farag SS, Lin TS, Avalos BR et al. High disease burden is associated with poor outcomes for patients with acute myeloid leukemia not in remission who undergo unrelated donor cell transplantation. Biol Blood Marrow Tr 2006; 12: 61–67.
Bishop MR, Alyea EP 3rd, Cairo MS, Falkenburg JH, June CH, Kroger N et al. National Cancer Institute's First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: summary and recommendations from the organizing committee. Biol Blood Marrow Transplant 2011; 17: 443–454.
Flynn CM, Hirsch B, Defor T, Barker JN, Miller JS, Wagner JE et al. Reduced intensity compared with high dose conditioning for allotransplantation in acute myeloid leukemia and myelodysplastic syndrome: a comparative clinical analysis. Am J Hematol 2007; 82: 867–872.
Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia 2005; 19: 2304–2312.
Alyea EP, Kim HT, Ho V, Cutler C, DeAngelo DJ, Stone R et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant 2006; 12: 1047–1055.
Alyea EP, Kim HT, Ho V, Cutler C, Gribben J, DeAngelo DJ et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood 2005; 105: 1810–1814.
Shimoni A, Hardan I, Shem-Tov N, Yeshurun M, Yerushalmi R, Avigdor A et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia 2006; 20: 322–328.
Martino R, Iacobelli S, Brand R, Jansen T, van Biezen A, Finke J et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood 2006; 108: 836–846.
Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol 2009; 27: 4570–4577.
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2003; 21: 4642–4649.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919.
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000; 96: 4075–4083.
Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood 2007; 110: 3064–3070.
Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE . Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood 2003; 102: 1915–1919.
Dunning K, Safo AO . The ultimate Wright-Giemsa stain: 60 years in the making. Biotechnol Histochem 2011; 86: 69–75.
Kaplan EL, Meier P . Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc 1958; 53: 457–481.
Lin DY . Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med 1997; 16: 901–910.
Cox DR . Regression models and life tables. J R Stat Soc Bull 1972; 34: 187–220.
Fine JP, Gray RJ . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
Paietta E . Minimal residual disease in acute myeloid leukemia: coming of age. Hematology Am Soc Hematol Educ Program 2012; 2012: 35–42.
Oran B, Popat U, Rondon G, Ravandi F, Garcia-Manero G, Abruzzo L et al. Significance of persistent cytogenetic abnormalities on myeloablative allogeneic stem cell transplantation in first complete remission. Biol Blood Marrow Transplant 2013; 19: 214–220.
Engel H, Drach J, Keyhani A, Jiang S, Van NT, Kimmel M et al. Quantitation of minimal residual disease in acute myelogenous leukemia and myelodysplastic syndromes in complete remission by molecular cytogenetics of progenitor cells. Leukemia 1999; 13: 568–577.
Kwon M, Martinez-Laperche C, Infante M, Carretero F, Balsalobre P, Serrano D et al. Evaluation of minimal residual disease by real-time quantitative PCR of Wilms' tumor 1 expression in patients with acute myelogenous leukemia after allogeneic stem cell transplantation: correlation with flow cytometry and chimerism. Biol Blood Marrow Transplant 2012; 18: 1235–1242.
Perea G, Lasa A, Aventin A, Domingo A, Villamor N, de Llano MPQ et al. Prognostic value of minimal residual disease (MRD) in acute myeloid leukemia (AML) with favorable cytogenetics [t(8;21) and inv(16)]. Leukemia 2006; 20: 87–94.
Jourdan E, Boissel N, Chevret S, Delabesse E, Renneville A, Cornillet P et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood 2013; 121: 2213–2223.
Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol 2011; 29: 1190–1197.
Loken MR, Alonzo TA, Pardo L, Gerbing RB, Raimondi SC, Hirsch BA et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children's Oncology Group. Blood 2012; 120: 1581–1588.
Kamble RT, Hjortsvang E, Selby GB . Leukemia burden and outcome of allogeneic transplant in acute myelogenous leukemia. Biol Blood Marrow Transplant 2006; 12: 691–692.
Gyurkocza B, Storb R, Storer BE, Chauncey TR, Lange T, Shizuru JA et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol 2010; 28: 2859–2867.
Oran B, Giralt S, Saliba R, Hosing C, Popat U, Khouri I et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biol Blood Marrow Transplant 2007; 13: 454–462.
Sayer HG, Kroger M, Beyer J, Kiehl M, Klein SA, Schaefer-Eckart K et al. Reduced intensity conditioning for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia: disease status by marrow blasts is the strongest prognostic factor. Bone Marrow Transplant 2003; 31: 1089–1095.
Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 2011; 118: 1402–1412.
Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo XH et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C(+) natural killer cells with potent function. Blood 2012; 119: 2665–2674.
Ossenkoppele G, Schuurhuis GJ . MRD in AML: time for redefinition of CR? Blood 2013; 121: 2166–2168.
Inaba H, Coustan-Smith E, Cao X, Pounds SB, Shurtleff SA, Wang KY et al. Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J Clin Oncol 2012; 30: 3625–3632.
Burke MJ, Burns L, Linden MA, Lindgren B, Verneris MR, Weisdorf D et al. How do we define complete remission for acute myeloid leukemia in the current era? Results of an international survey. Am J Hematol, (e-pub ahead of print 4 June 2013; doi:10.1002/ajh.23497).
Leung W, Pui CH, Coustan-Smith E, Yang J, Pei D, Gan K et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood 2012; 120: 468–472.
Wayne AS, Radich JP . Pretransplant MRD: the light is yellow, not red. Blood 2012; 120: 244–246.
Kim DY, Lee JH, Park YH, Lee JH, Kim SD, Choi Y et al. Feasibility of hypomethylating agents followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome. Bone Marrow Transplant 2012; 47: 374–379.
Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005; 105: 3051–3057.
Warlick ED, Tomblyn M, Cao Q, Defor T, Blazar BR, Macmillan M et al. Reduced-intensity conditioning followed by related allografts in hematologic malignancies: long-term outcomes most successful in indolent and aggressive non-Hodgkin lymphomas. Biol Blood Marrow Transplant 2011; 17: 1025–1032.
Tallman MS, Rowlings PA, Milone G, Zhang MJ, Perez WS, Weisdorf D et al. Effect of postremission chemotherapy before human leukocyte antigen-identical sibling transplantation for acute myelogenous leukemia in first complete remission. Blood 2000; 96: 1254–1258.
Buckley SA, Appelbaum FR, Walter RB . Prognostic and therapeutic implications of minimal residual disease at the time of transplantation in acute leukemia. Bone Marrow Transplant 2013; 48: 630–641.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ustun, C., Wiseman, A., DeFor, T. et al. Achieving stringent CR is essential before reduced-intensity conditioning allogeneic hematopoietic cell transplantation in AML. Bone Marrow Transplant 48, 1415–1420 (2013). https://doi.org/10.1038/bmt.2013.124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.124
Keywords
This article is cited by
-
Measurable residual disease (MRD) status before allogeneic hematopoietic cell transplantation impact on secondary acute myeloid leukemia outcome. A Study from the Acute Leukemia Working Party (ALWP) of the European society for Blood and Marrow Transplantation (EBMT)
Bone Marrow Transplantation (2022)
-
Importance of conditioning regimen intensity, MRD positivity, and KIR ligand mismatch in UCB transplantation
Bone Marrow Transplantation (2018)
-
Effects of post-remission chemotherapy before allo-HSCT for acute myeloid leukemia during first complete remission: a meta-analysis
Annals of Hematology (2018)
-
Minimal residual disease by either flow cytometry or cytogenetics prior to an allogeneic hematopoietic stem cell transplant is associated with poor outcome in acute myeloid leukemia
Blood Cancer Journal (2017)
-
Novel disease burden assessment predicts allogeneic transplantation outcomes in myelodysplastic syndrome
Bone Marrow Transplantation (2016)