Abstract
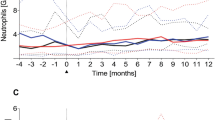
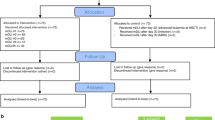
The number of survivors having undergone high-dose therapy (HDT) followed by auto-SCT continues to increase, although some of the long-term sequelae remain incompletely understood. The immunological status and quality of life of 37 HDT/auto-SCT survivors with lymphoma in continuous remission of ⩾3 years were assessed alongside 14 age-matched controls. At a median follow-up of 10.5 years (range 2.2–20.2) following HDT/auto-SCT, the proportion of CD4+ cells remained significantly reduced in patients compared with controls (median 43.4% vs 62.5%, respectively; P=<0.001), predominantly a result of sustained reduction in the naive CD4+ component (P<0.001). Naive CD8+ lymphocytes (P=0.014) and transitional B cells (P=0.008) were also significantly reduced, but differences in other lymphocyte subsets were not observed. Uptake of revaccination following HDT/auto-SCT was sporadic; between 11% and 33% of patients had serological titres outside the protective ranges for five of six routinely used vaccines. In the main, patients were found to have a good quality of life, although their EORTC QLQ-C30 questionnaire scores were significantly lower for the physical and social functioning domains compared with controls. Ten years after HDT/auto-SCT immunological deficits persist; to avoid excess risk of preventable disease, serological immunity should be assessed post HDT/auto-SCT followed by appropriate revaccination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med 1995; 333: 1540–1545.
Schouten H, Qian W, Kvaloy S, Porcellini A, Hagberg H, Johnson H et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin's lymphoma: results from the randomized European CUP trial. J Clin Oncol 2003; 21: 3918–3927.
Linch D, Winfield D, Goldstone A, Moir D, Hancock B, McMillan A et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet 1993; 341: 1051–1054.
Schmitz N, Pfistner B, Sextro M, Sieber M, Carella A, Haenel M et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet 2002; 359: 2065–2071.
Kiesel S, Pezzutto A, Moldenhauer G, Haas R, Körbling M, Hunstein W et al. B-cell proliferative and differentiative responses after autologous peripheral blood stem cell or bone marrow transplantation. Blood 1988; 72: 672–678.
Bomberger C, Singh-Jairam M, Rodey G, Guerriero A, Yeager A, Fleming W et al. Lymphoid reconstitution after autologous PBSC transplantation with FACS-sorted CD34+ hematopoietic progenitors. Blood 1998; 91: 2588–2600.
Pedrazzini A, Freedman A, Andersen J, Heflin L, Anderson K, Takvorian T et al. Anti-B-cell monoclonal antibody-purged autologous bone marrow transplantation for B-cell non-Hodgkin's lymphoma: phenotypic reconstitution and B-cell function. Blood 1989; 74: 2203–2211.
Nordøy T, Kolstad A, Endresen P, Holte H, Kvaløy S, Kvalheim G et al. Persistent changes in the immune system 4–10 years after ABMT. Bone Marrow Transplant 1999; 24: 873–878.
Hammarström V, Pauksen K, Azinge J, Oberg G, Ljungman P . Pneumococcal immunity and response to immunization with pneumococcal vaccine in bone marrow transplant patients: the influence of graft vs host reaction. Support Care Cancer 1993; 1: 195–199.
Pauksen K, Hammarström V, Ljungman P, Sjölin J, Oberg G, Lönnerholm G et al. Immunity to poliovirus and immunization with inactivated poliovirus vaccine after autologous bone marrow transplantation. Clin Infect Dis 1994; 18: 547–552.
Hammarström V, Pauksen K, Björkstrand B, Simonsson B, Oberg G, Ljungman P . Tetanus immunity in autologous bone marrow and blood stem cell transplant recipients. Bone Marrow Transplant 1998; 22: 67–71.
Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm Rep 2000; 49: 1–125 CE1-7.
Rizzo J, Wingard J, Tichelli A, Lee S, Van Lint M, Burns L et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2006; 12: 138–151.
Ljungman P, Engelhard D, de la Cámara R, Einsele H, Locasciulli A, Martino R et al. Vaccination of stem cell transplant recipients: recommendations of the Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant 2005; 35: 737–746.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376.
Kemmler G, Holzner B, Kopp M, Dünser M, Greil R, Hahn E et al. Multidimensional scaling as a tool for analysing quality of life data. Qual Life Res 2002; 11: 223–233.
Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A . The EORTC QLQ-C30 Scoring Manual, 3rd edn. European Organisation for Research and Treatment of Cancer: Brussels, 2001.
Heitger A, Neu N, Kern H, Panzer-Grümayer E, Greinix H, Nachbaur D et al. Essential role of the thymus to reconstitute naive (CD45RA+) T-helper cells after human allogeneic bone marrow transplantation. Blood 1997; 90: 850–857.
Douek D, Vescio R, Betts M, Brenchley J, Hill B, Zhang L et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet 2000; 355: 1875–1881.
Poulin J, Sylvestre M, Champagne P, Dion M, Kettaf N, Dumont A et al. Evidence for adequate thymic function but impaired naive T-cell survival following allogeneic hematopoietic stem cell transplantation in the absence of chronic graft-versus-host disease. Blood 2003; 102: 4600–4607.
Sportès C, Hakim F, Memon S, Zhang H, Chua K, Brown M et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med 2008; 205: 1701–1714.
Syrjala K, Langer S, Abrams J, Storer B, Sanders J, Flowers M et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA 2004; 291: 2335–2343.
Acknowledgements
This work was supported by Cancer Research UK, Southampton Experimental Cancer Medicines Centre, University of Southampton, Faculty of Medicine and Southampton University Hospitals Trust. We are indebted to the volunteers who gave up their time to participate in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Dean, H., Cazaly, A., Hurlock, C. et al. Defects in lymphocyte subsets and serological memory persist a median of 10 years after high-dose therapy and autologous progenitor cell rescue for malignant lymphoma. Bone Marrow Transplant 47, 1545–1551 (2012). https://doi.org/10.1038/bmt.2012.73
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.73
Keywords
This article is cited by
-
Patient-reported cognitive function among hematopoietic stem cell transplant and cellular therapy patients: a scoping review
Quality of Life Research (2023)