Abstract
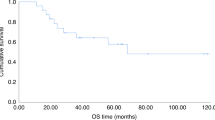
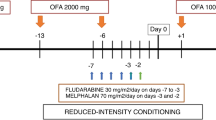
A total of 20 patients enrolled in a multicenter phase II dose escalation study of radioimmunotherapy (RIT) using yttrium-90-ibritumomab tiuxetan at two dose levels (22 and 30 MBq/kg) in 10 patients, combined with reduced intensity conditioning (RIC) using fludarabine, melphalan and alemtuzumab followed by allogeneic hematopoietic cell transplantation (HCT) from either matched-related (n=5) or matched-unrelated donors (n=15). Postgrafting immunosuppression with cyclosporine was administered. Diagnoses were diffuse large B-cell lymphoma (n=13), transformed CLL (n=4), blastic mantle cell lymphoma (n=2) and follicular lymphoma grade 3 (n=1). Median age was 51 (range, 29–69) years. All patients were high risk with relapsed/refractory disease or relapse after preceding autologous HCT. Median follow-up of patients alive was 1115 (range, 1006–1252) days. No directly RIT-related toxicities were observed. The cumulative incidence of non-relapse mortality was 30%. Incidences of grade II–IV acute and chronic GvHD was 45% and 70%, respectively. Kaplan–Meier estimated 3-year OS and EFS were 20% for both dose levels. In conclusion, dose escalation of RIT and combined use with RIC is feasible with no additional toxicity due to dose escalation. This study is registered on http://clinicaltrials.gov as NCT00302757.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hamadani M, Benson DM, Hofmeister CC, Elder P, Blum W, Porcu P et al. Allogeneic stem cell transplantation for patients with relapsed chemorefractory aggressive non-hodgkin lymphomas. Biol Blood Marrow Transplant 2009; 15: 547–553.
Peniket AJ, Ruiz De Elvira MC, Taghipour G, Cordonnier C, Gluckman E, de Witte T et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant 2003; 31: 667–678.
Tse WW, Lazarus HM, Van Besien K . Stem cell transplantation in follicular lymphoma: progress at last? Bone Marrow Transplant 2004; 34: 929–938.
Verdonck LF, Dekker AW, Lokhorst HM, Petersen EJ, Nieuwenhuis HK . Allogeneic versus autologous bone marrow transplantation for refractory and recurrent low-grade non-Hodgkin’s lymphoma. Blood 1997; 90: 4201–4205.
Sandmaier BM, McSweeney P, Yu C, Storb R . Nonmyeloablative transplants: preclinical and clinical results. Semin Oncol 2000; 27: 78–81.
Morris E, Thomson K, Craddock C, Mahendra P, Milligan D, Cook G et al. Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood 2004; 104: 3865–3871.
Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non- Hodgkin’s lymphoma. J Clin Oncol 2002; 20: 2453–2463.
Wang M, Oki Y, Pro B, Romaguera JE, Rodriguez MA, Samaniego F et al. Phase II study of yttrium-90-ibritumomab tiuxetan in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol 2009; 27: 5213–5218.
Jain N, Wierda W, Ferrajoli A, Wong F, Lerner S, Keating M et al. A phase 2 study of yttrium-90 ibritumomab tiuxetan (Zevalin) in patients with chronic lymphocytic leukemia. Cancer 2009; 115: 4533–4539.
Morschhauser F, Radford J, Van Hoof A, Vitolo U, Soubeyran P, Tilly H et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol 2008; 26: 5156–5164.
Bader P, Beck J, Frey A, Schlegel PG, Hebarth H, Handgretinger R et al. Serial and quantitative analysis of mixed hematopoietic chimerism by PCR in patients with acute leukemias allows the prediction of relapse after allogeneic BMT. Bone Marrow Transplant 1998; 21: 487–495.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–586.
Sullivan KM . Graft-versus-host-disease. In: Blume KG, Forman SJ, Appelbaum FR eds.. Thomas’ Hematopoietic Cell Transplantation. Blackwell Science Ltd, Oxford: UK. 2004, pp 635–664.
R Development Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2011.
Scrucca L, Santucci A, Aversa F . Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant 2007; 40: 381–387.
Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de Witte T et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer 2009; 115: 4715–4726.
Tennvall J, Fischer M, Bischof DA, Bombardieri E, Bodei L, Giammarile F et al. EANM procedure guideline for radio-immunotherapy for B-cell lymphoma with 90Y-radiolabelled ibritumomab tiuxetan (Zevalin). Eur J Nucl Med Mol Imaging 2007; 34: 616–622.
Witzig TE, White CA, Wiseman GA, Gordon LI, Emmanouilides C, Raubitschek A et al. Phase I/II trial of IDEC-Y2B8 radioimmunotherapy for treatment of relapsed or refractory CD20(+) B-cell non-Hodgkin’s lymphoma. J Clin Oncol 1999; 17: 3793–3803.
Witzig TE, Flinn IW, Gordon LI, Emmanouilides C, Czuczman MS, Saleh MN et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol 2002; 20: 3262–3269.
Emmanouilides C, Witzig TE, Gordon LI, Vo K, Wiseman GA, Flinn IW et al. Treatment with yttrium 90 ibritumomab tiuxetan at early relapse is safe and effective in patients with previously treated B-cell non-Hodgkin’s lymphoma. Leuk Lymphoma 2006; 47: 629–636.
Morschhauser F, Illidge T, Huglo D, Martinelli G, Paganelli G, Zinzani PL et al. Efficacy and safety of yttrium-90 ibritumomab tiuxetan in patients with relapsed or refractory diffuse large B-cell lymphoma not appropriate for autologous stem-cell transplantation. Blood 2007; 110: 54–58.
Wiseman GA, Kornmehl E, Leigh B, Erwin WD, Podoloff DA, Spies S et al. Radiation dosimetry results and safety correlations from 90Y-ibritumomab tiuxetan radioimmunotherapy for relapsed or refractory non-Hodgkin’s lymphoma: combined data from 4 clinical trials. J Nucl Med 2003; 44: 465–474.
Krishnan A, Nademanee A, Fung HC, Raubitschek AA, Molina A, Yamauchi D et al. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin’s lymphoma. J Clin Oncol 2008; 26: 90–95.
Press OW, Eary JF, Gooley T, Gopal AK, Liu S, Rajendran JG et al. A phase I/II trial of iodine-131-tositumomab (anti-CD20), etoposide, cyclophosphamide, and autologous stem cell transplantation for relapsed B-cell lymphomas. Blood 2000; 96: 2934–2942.
Vose JM, Bierman PJ, Enke C, Hankins J, Bociek G, Lynch JC et al. Phase I trial of iodine-131 tositumomab with high-dose chemotherapy and autologous stem-cell transplantation for relapsed non-Hodgkin’s lymphoma. J Clin Oncol 2005; 23: 461–467.
Decaudin D, Mounier N, Tilly H, Ribrag V, Ghesquieres H, Bouabdallah K et al. (90)Y ibritumomab tiuxetan (Zevalin) combined with BEAM (Z-BEAM) conditioning regimen plus autologous stem cell transplantation in relapsed or refractory low-grade CD20-positive B-cell lymphoma. A GELA Phase II Prospective Study. Clin Lymphoma Myeloma Leuk 2011; 11: 212–218.
Winter JN, Inwards DJ, Spies S, Wiseman G, Patton D, Erwin W et al. Yttrium ibritumomab tiuxetan doses calculated to deliver up to 15 Gy to critical organs may be safely combined with high-dose BEAM and autologous transplantation in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol 2009; 27: 1653–1659.
Devizzi L, Guidetti A, Tarella C, Magni M, Matteucci P, Seregni E et al. High-dose yttrium-90-ibritumomab tiuxetan with tandem stem-cell reinfusion: an outpatient preparative regimen for autologous hematopoietic cell transplantation. J Clin Oncol 2008; 26: 5175–5182.
Shimoni A, Zwas ST, Oksman Y, Hardan I, Shem-Tov N, Rand A et al. Ibritumomab tiuxetan (Zevalin) combined with reduced-intensity conditioning and allogeneic stem-cell transplantation (SCT) in patients with chemorefractory non-Hodgkin’s lymphoma. Bone Marrow Transplant 2008; 41: 355–361.
Fietz T, Uharek L, Gentilini C, Muessig A, Rieger K, Marinets O et al. Allogeneic hematopoietic cell transplantation following conditioning with 90Y-ibritumomab-tiuxetan. Leuk Lymphoma 2006; 47: 59–63.
Bethge WA, Lange T, Meisner C, von Harsdorf S, Bornhaeuser M, Federmann B et al. Radioimmunotherapy with yttrium-90-ibritumomab tiuxetan as part of a reduced- intensity conditioning regimen for allogeneic hematopoietic cell transplantation in patients with advanced non-Hodgkin lymphoma: results of a phase 2 study. Blood 2010; 116: 1795–1802.
Gopal AK, Guthrie KA, Rajendran J, Pagel JM, Oliveira G, Maloney DG et al. 90Y-ibritumomab tiuxetan, fludarabine, and TBI based non-myeloablative allogeneic transplant conditioning for patients with persistent high-risk B-cell lymphoma. Blood 2011; 118: 1132–1139.
Wiseman GA, White CA, Stabin M, Dunn WL, Erwin W, Dahlbom M et al. Phase I/II 90Y-Zevalin (yttrium-90 ibritumomab tiuxetan, IDEC-Y2B8) radioimmunotherapy dosimetry results in relapsed or refractory non-Hodgkin’s lymphoma. Eur J Nucl Med 2000; 27: 766–777.
Chiesa C, Botta F, Coliva A, Maccauro M, Devizzi L, Guidetti A et al. Absorbed dose and biologically effective dose in patients with high-risk non-Hodgkin’s lymphoma treated with high-activity myeloablative 90Y-ibritumomab tiuxetan (Zevalin). Eur J Nucl Med Mol Imaging 2009; 36: 1745–1757.
Rezvani AR, Storer B, Maris M, Sorror ML, Agura E, Maziarz RT et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-hodgkin’s lymphoma. J Clin Oncol 2008; 26: 211–217.
van Kampen RJ, Canals C, Schouten HC, Nagler A, Thomson KJ, Vernant JP et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin’s lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol 2011; 29: 1342–1348.
Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE et al. Impact of immune modulation with anti-T cell antibodies on outcomes of reduced intensity allogeneic hematopoietic cell transplantation for hematologic malignancies. Blood 2011; 117: 6963–6970.
Thomson KJ, Morris EC, Milligan D, Parker AN, Hunter AE, Cook G et al. T-cell-depleted reduced-intensity transplantation followed by donor leukocyte infusions to promote graft-versus-lymphoma activity results in excellent long-term survival in patients with multiply relapsed follicular lymphoma. J Clin Oncol 2010; 28: 3695–3700.
Rezvani AR, Norasetthada L, Gooley T, Sorror M, Bouvier ME, Sahebi F et al. Non-myeloablative allogeneic haematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: a multicentre experience. Br J Haematol 2008; 143: 395–403.
Acknowledgements
We thank the nurses and staff of the transplant units taking part in the study. We also thank the departments of nuclear medicine participating in the study, in particularly R Bares, Tuebingen, SN Reske, Ulm and J Kotzerke, Dresden. Furthermore, we acknowledge Dagmar Frisch and Anja Junker for data management and monitoring of the study. This study was funded by the German José Carreras Foundation and an unrestricted research grant by Bayer Health Care GmbH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Wolfgang A Bethge received honoraria and research funding from the Bayer Health Care GmbH, Martin Bornhauser received honoraria from Hoffmann La Roche, Novartis and Celgene, and research funding from Hoffmann La Roche and Novartis, rest of the authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Bone Marrow Transplantation website
Rights and permissions
About this article
Cite this article
Bethge, W., von Harsdorf, S., Bornhauser, M. et al. Dose-escalated radioimmunotherapy as part of reduced intensity conditioning for allogeneic transplantation in patients with advanced high-grade non-Hodgkin lymphoma. Bone Marrow Transplant 47, 1397–1402 (2012). https://doi.org/10.1038/bmt.2012.62
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.62
Keywords
This article is cited by
-
Accelerated therapeutic progress in diffuse large B cell lymphoma
Annals of Hematology (2014)
-
The role of allogeneic haematopoietic progenitor cell transplantation in patients with diffuse large B-cell non-Hodgkin lymphomas (DLBCL)
Bone Marrow Transplantation (2013)