Abstract
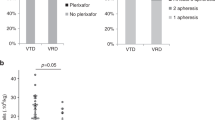
Lenalidomide is associated with suboptimal autologous hematopoietic stem cell (AHSC) mobilization. We hypothesized that growth factor plus preemptive plerixafor is an effective strategy for AHSC mobilization in multiple myeloma (MM) despite prior exposure to lenalidomide. We retrospectively reviewed patient characteristics and mobilization outcomes of 89 consecutive MM patients undergoing first mobilization with filgrastim or pegfilgrastim +/− preemptive plerixafor using a previously validated algorithm based on day 4 peripheral blood CD34+ cell count (PB-CD34+) and mobilization target. Outcomes were analyzed according to the extent of prior exposure to lenalidomide: no prior exposure (group A, n=40), 1– 4 cycles (group B, n=30) and >4 cycles (group C, n=19). Multivariate analysis yielded only age and number of cycles of lenalidomide as negatively associated, and mobilization with pegfilgrastim as positively associated with higher PB-CD34+. Only 45% of patients in group A required plerixafor vs 63% in groups B and 84% in C, P=0.01. A higher proportion of patients in group A (100%) met the mobilization target than in groups B (90%) or C (79%), P=0.008. All patients yielded at least 2 × 106 CD34+/kg. Growth factor mobilization with preemptive plerixafor is an adequate upfront mobilization strategy for MM patients regardless of prior exposure to lenalidomide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335: 91–97.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–1883.
Fermand JP, Katsahian S, Divine M, Leblond V, Dreyfus F, Macro M et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the group myelome-autogreffe. J Clin Oncol 2005; 23: 9227–9233.
Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol 2010; 11: 29–37.
Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010; 116: 679–686.
Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol 2010; 28: 4621–4629.
Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia 2007; 21: 2035–2042.
Mazumder A, Kaufman J, Niesvizky R, Lonial S, Vesole D, Jagannath S . Effect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patients. Leukemia 2008; 22: 1280–1281; author reply 1281–1282.
Paripati H, Stewart AK, Cabou S, Dueck A, Zepeda VJ, Pirooz N et al. Compromised stem cell mobilization following induction therapy with lenalidomide in myeloma. Leukemia 2008; 22: 1282–1284.
Popat U, Saliba R, Thandi R, Hosing C, Qazilbash M, Anderlini P et al. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant 2009; 15: 718–723.
Cavallo F, Bringhen S, Milone G, Ben-Yehuda D, Nagler A, Calabrese E et al. Stem cell mobilization in patients with newly diagnosed multiple myeloma after lenalidomide induction therapy. Leukemia 2011; 25: 1627–1631.
Mark T, Stern J, Furst JR, Jayabalan D, Zafar F, LaRow A et al. Stem cell mobilization with cyclophosphamide overcomes the suppressive effect of lenalidomide therapy on stem cell collection in multiple myeloma. Biol Blood Marrow Transplant 2008; 14: 795–798.
Nazha A, Cook R, Vogl DT, Mangan PA, Gardler M, Hummel K et al. Stem cell collection in patients with multiple myeloma: impact of induction therapy and mobilization regimen. Bone Marrow Transplant 2011; 46: 59–63.
Costa LJ, Miller AN, Alexander ET, Hogan KR, Shabbir M, Schaub C et al. Growth factor and patient-adapted use of plerixafor is superior to CY and growth factor for autologous hematopoietic stem cells mobilization. Bone Marrow Transplant 2011; 46: 523–528.
Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzow MR et al. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma 2006; 6: 384–388.
Schwartzberg LS, Birch R, Hazelton B, Tauer KW, Lee P, Altemose R et al. Peripheral blood stem cell mobilization by chemotherapy with and without recombinant human granulocyte colony-stimulating factor. J Hematother 1992; 1: 317–327.
Shaughnessy P, Islas-Ohlmayer M, Murphy J, Hougham M, Macpherson J, Winkler K et al. Cost and clinical analysis of autologous hematopoietic stem cell mobilization with G-CSF and plerixafor compared to G-CSF and cyclophosphamide. Biol Blood Marrow Transplant 2011; 17: 729–736.
DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 2009; 113: 5720–5726.
Malard F, Kroger N, Gabriel IH, Hubel K, Apperley JF, Basak GW et al. Plerixafor for autologous peripheral blood stem cell mobilization in patients previously treated with fludarabine or lenalidomide. Biol Blood Marrow Transplant 2012; 18: 314–317.
Micallef IN, Ho AD, Klein LM, Marulkar S, Gandhi PJ, McSweeney PA . Plerixafor (Mozobil) for stem cell mobilization in patients with multiple myeloma previously treated with lenalidomide. Bone Marrow Transplant 2010; 46: 350–355.
Sinha S, Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK et al. Majority of patients receiving initial therapy with lenalidomide-based regimens can be successfully mobilized with appropriate mobilization strategies. Leukemia 2012; 26: 1119–1122.
Costa LJ, Alexander ET, Hogan KR, Schaub C, Fouts TV, Stuart RK . Development and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilization. Bone Marrow Transplant 2011; 46: 64–69.
Costa LJ, Hogan KR, Kramer C, Butcher C, Littleton A, Shoptaw KB et al. Comparison between pegfilgrastim and filgrastim-based autologous hematopoietic stem cell mobilization in the setting of patient adapted (‘just in time’) plerixafor: efficacy and cost analysis. Blood 2011; 118 (abstract 1921).
Costa LJ, Miller AN, Alexander ET, Hogan KR, Shabbir M, Schaub C et al. Growth factor and patient-adapted use of plerixafor is superior to CY and growth factor for autologous hematopoietic stem cells mobilization. Bone Marrow Transplant 2011; 46: 523–528.
Alexander ET, Towery JA, Miller AN, Kramer C, Hogan KR, Squires JE et al. Beyond CD34+ cell dose: impact of method of peripheral blood hematopoietic stem cell mobilization (granulocyte-colony-stimulating factor [G-CSF], G-CSF plus plerixafor, or cyclophosphamide G-CSF/granulocyte-macrophage [GM]-CSF) on number of colony-forming unit-GM, engraftment, and day +100 hematopoietic graft function. Transfusion 2011; 51: 1995–2000.
Sinha S, Gastineau D, Micallef I, Hogan W, Ansell S, Buadi F et al. Predicting PBSC harvest failure using circulating CD34 levels: developing target-based cutoff points for early intervention. Bone Marrow Transplant 2011; 46: 943–949.
Abhyankar S, Dejarnette S, Aljitawi O, Ganguly S, Merkel D, McGuirk J . A risk-based approach to optimize autologous hematopoietic stem cell (HSC) collection with the use of plerixafor. Bone Marrow Transplant 2012; 47: 483–487.
Li J, Hamilton E, Vaughn L, Graiser M, Renfroe H, Lechowicz MJ et al. Effectiveness and cost analysis of ‘just-in-time’ salvage plerixafor administration in autologous transplant patients with poor stem cell mobilization kinetics. Transfusion 2011; 51: 2175–2182.
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008; 111: 2516–2520.
Palumbo A, Bringhen S, Petrucci MT, Musto P, Rossini F, Nunzi M et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood 2004; 104: 3052–3057.
Boccadoro M, Palumbo A, Bringhen S, Merletti F, Ciccone G, Richiardi L et al. Oral melphalan at diagnosis hampers adequate collection of peripheral blood progenitor cells in multiple myeloma. Haematologica 2002; 87: 846–850.
Goldschmidt H, Hegenbart U, Wallmeier M, Hohaus S, Haas R . Factors influencing collection of peripheral blood progenitor cells following high-dose cyclophosphamide and granulocyte colony-stimulating factor in patients with multiple myeloma. Br J Haematol 1997; 98: 736–744.
Jansen J, Thompson J, Dugan M, Wiemann M, Hanks S, Greenspan A et al. Impaired PBPC collection in patients with myeloma after high-dose melphalan. Cytotherapy 2004; 6: 498–504.
Davies F, Baz R . Lenalidomide mode of action: linking bench and clinical findings. Blood Rev 2010; 24(Suppl 1): S13–S19.
Desikan KR, Barlogie B, Jagannath S, Vesole DH, Siegel D, Fassas A et al. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. J Clin Oncol 1998; 16: 1547–1553.
Stiff P, Micallef I, McCarthy P, Magalhaes-Silverman M, Weisdorf D, Territo M et al. Treatment with plerixafor in non-Hodgkin's lymphoma and multiple myeloma patients to increase the number of peripheral blood stem cells when given a mobilizing regimen of G-CSF: implications for the heavily pretreated patient. Biol Blood Marrow Transplant 2009; 15: 249–256.
Tricot G, Cottler-Fox MH, Calandra G . Safety and efficacy assessment of plerixafor in patients with multiple myeloma proven or predicted to be poor mobilizers, including assessment of tumor cell mobilization. Bone Marrow Transplant 2010; 45: 63–68.
Micallef I, Sinha S, Gastineau D, Wolf R, Inwards D, Gertz M et al. A cost effective analysis of a risk-adapted algorithm for plerixafor use in autologous peripheral blood stem cell mobilization. Biol Blood Marrow Transplant 2011; 17: S159–S160.
Vishnu P, Roy V, Paulsen A, Zubair AC . Efficacy and cost-benefit analysis of risk-adaptive use of plerixafor for autologous hematopoietic progenitor cell mobilization. Transfusion 2012; 52: 55–62.
Anwer F, Green MR, Yeager AM . Effective primary mobilization of autologous peripheral blood stem cells with granulocyte colony-stimulating factor and plerixafor in lenalidomide-treated patients with multiple myeloma. ASH Annu Meet Abstr 2010; 116: 2254.
Schulman KA, Birch R, Zhen B, Pania N, Weaver CH . Effect of CD34(+) cell dose on resource utilization in patients after high-dose chemotherapy with peripheral-blood stem-cell support. J Clin Oncol 1999; 17: 1227.
Scheid C, Draube A, Reiser M, Schulz A, Chemnitz J, Nelles S et al. Using at least 5 × 10(6)/kg CD34+ cells for autologous stem cell transplantation significantly reduces febrile complications and use of antibiotics after transplantation. Bone Marrow Transplant 1999; 23: 1177–1181.
Lefrere F, Delarue R, Somme D, Levy V, Damaj G, Tu A et al. High-dose CD34+ cells are not clinically relevant in reducing cytopenia and blood component consumption following myeloablative therapy and peripheral blood progenitor cell transplantation as compared with standard dose. Transfusion 2002; 42: 443–450.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
LJC received honorarium from Sanofi-Genzyme. The other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Costa, L., Abbas, J., Hogan, K. et al. Growth factor plus preemptive (‘just-in-time’) plerixafor successfully mobilizes hematopoietic stem cells in multiple myeloma patients despite prior lenalidomide exposure. Bone Marrow Transplant 47, 1403–1408 (2012). https://doi.org/10.1038/bmt.2012.60
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.60
Keywords
This article is cited by
-
Impact of body mass index (BMI) on the efficacy of plerixafor for hematopoietic progenitor cell (HPC) mobilization
Bone Marrow Transplantation (2022)
-
Results from a multicenter, noninterventional registry study for multiple myeloma patients who received stem cell mobilization regimens with and without plerixafor
Bone Marrow Transplantation (2020)
-
The timing of plerixafor addition to G-Csf and chemotherapy affects immunological recovery after autologous stem cell transplant in multiple myeloma
Bone Marrow Transplantation (2020)
-
Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: a Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus statement
Bone Marrow Transplantation (2019)
-
A randomized phase II study of stem cell mobilization with cyclophosphamide+G-CSF or G-CSF alone after lenalidomide-based induction in multiple myeloma
Bone Marrow Transplantation (2016)