Abstract
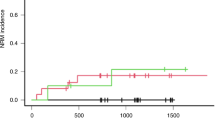
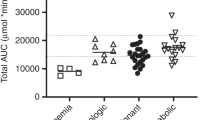
BU is a key compound of conditioning regimens in children undergoing hematopoietic SCT (HSCT). Inter-individual differences in BU pharmacokinetics (PKs) might affect BU efficacy and toxicity. As BU is mainly metabolized by glutathione S-transferase (GST), we investigated the relationship between GSTA1, GSTM1 and GSTP1 genotypes with first-dose BU PKs, and the relationship with HSCT outcomes in 69 children receiving myeloablative conditioning regimen. GSTM1 null genotype correlated with higher BU exposure and lower clearance in patients older than 4 years (P⩽0.04). In accordance with the suggested functional role, GSTA1*A2 haplotype was associated with lower drug levels and higher drug clearance (P⩽0.03). Gene-dosage effect was also observed (P⩽0.007). GSTA1 haplotypes were associated with HSCT outcomes. Patients with two copies of haplotype *A2 had better event free survival (P=0.03). In contrast, homozygous individuals for haplotypes *B and *B1 had higher occurrence of veno-occlusive disease (P=0.009). GSTM1 null individuals older than 4 years had more frequently graft versus host disease (P=0.03). In conclusion, we showed that GST gene variants influence BU PK and outcomes of HSCT in children. A model for the dosage adjustment with the inclusion of genetic and non-genetic factors should be evaluated in a future prospective validation cohort.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood 1997; 89: 3055–3060.
Dalle JH, Wall D, Theoret Y, Duval M, Shaw L, Larocque D et al. Intravenous busulfan for allogeneic hematopoietic stem cell transplantation in infants: clinical and pharmacokinetic results. Bone Marrow Transplant 2003; 32: 647–651.
Tran H, Petropoulos D, Worth L, Mullen CA, Madden T, Andersson B et al. Pharmacokinetics and individualized dose adjustment of intravenous busulfan in children with advanced hematologic malignancies undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2004; 10: 805–812.
Vassal G, Michel G, Esperou H, Gentet JC, Valteau-Couanet D, Doz F et al. Prospective validation of a novel IV busulfan fixed dosing for paediatric patients to improve therapeutic AUC targeting without drug monitoring. Cancer Chemother Pharmacol 2008; 61: 113–123.
Bolinger AM, Zangwill AB, Slattery JT, Risler LJ, Sultan DH, Glidden DV et al. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation. Bone Marrow Transplant 2001; 28: 1013–1018.
McCune JS, Gibbs JP, Slattery JT . Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet 2000; 39: 155–165.
Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant 2002; 8: 477–485.
Malar R, Sjoo F, Rentsch K, Hassan M, Gungor T . Therapeutic drug monitoring is essential for intravenous busulfan therapy in pediatric hematopoietic stem cell recipients. Pediatr Transplant 2011; 15: 580–588.
Rowe JD, Nieves E, Listowsky I . Subunit diversity and tissue distribution of human glutathione S-transferases: interpretations based on electrospray ionization-MS and peptide sequence-specific antisera. Biochem J 1997; 325 (Part 2): 481–486.
Cooper AJ, Younis IR, Niatsetskaya ZV, Krasnikov BF, Pinto JT, Petros WP et al. Metabolism of the cysteine S-conjugate of busulfan involves a beta-lyase reaction. Drug Metab Dispos 2008; 36: 1546–1552.
Czerwinski M, Gibbs JP, Slattery JT . Busulfan conjugation by glutathione S-transferases alpha, mu, and pi. Drug Metab Dispos 1996; 24: 1015–1019.
Gulbis AM, Culotta KS, Jones RB, Andersson BS . Busulfan and metronidazole: an often forgotten but significant drug interaction. Ann Pharmacother 2011; 45: e39.
Nath CE, Earl JW, Pati N, Stephen K, Shaw PJ . Variability in the pharmacokinetics of intravenous busulphan given as a single daily dose to paediatric blood or marrow transplant recipients. Br J Clin Pharmacol 2008; 66: 50–59.
Schechter T, Finkelstein Y, Doyle J, Verjee Z, Moretti M, Koren G et al. Pharmacokinetic disposition and clinical outcomes in infants and children receiving intravenous busulfan for allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2007; 13: 307–314.
Ansari M, Lauzon-Joset JF, Vachon MF, Duval M, Theoret Y, Champagne MA et al. Influence of GST gene polymorphisms on busulfan pharmacokinetics in children. Bone Marrow Transplant 2010; 45: 261–267.
Bartelink IH, Boelens JJ, Bredius RG, Egberts AC, Wang C, Bierings MB et al. Body weight-dependent pharmacokinetics of busulfan in paediatric haematopoietic stem cell transplantation patients: towards individualized dosing. Clin Pharmacokinet 2012; 51: 331–345.
Elhasid R, Krivoy N, Rowe JM, Sprecher E, Adler L, Elkin H et al. Influence of glutathione S-transferase A1, P1, M1, T1 polymorphisms on oral busulfan pharmacokinetics in children with congenital hemoglobinopathies undergoing hematopoietic stem cell transplantation. Pediatr Blood Cancer 2010; 55: 1172–1179.
Paci A, Vassal G, Moshous D, Dalle JH, Bleyzac N, Neven B et al. Pharmacokinetic behavior and appraisal of intravenous busulfan dosing in infants and older children: the results of a population pharmacokinetic study from a large pediatric cohort undergoing hematopoietic stem-cell transplantation. Ther Drug Monit 2012; 34: 198–208.
Bartelink IH, Bredius RG, Belitser SV, Suttorp MM, Bierings M, Knibbe CA et al. Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematologic stem cell transplantation. Biol Blood Marrow Transplant 2009; 15: 231–241.
Ansari M, Krajinovic M . Can the pharmacogenetics of GST gene polymorphisms predict the dose of busulfan in pediatric hematopoietic stem cell transplantation? Pharmacogenomics 2009; 10: 1729–1732.
Bredschneider M, Klein K, Murdter TE, Marx C, Eichelbaum M, Nussler AK et al. Genetic polymorphisms of glutathione S-transferase A1, the major glutathione S-transferase in human liver: consequences for enzyme expression and busulfan conjugation. Clin Pharmacol Ther 2002; 71: 479–487.
Coles BF, Morel F, Rauch C, Huber WW, Yang M, Teitel CH et al. Effect of polymorphism in the human glutathione S-transferase A1 promoter on hepatic GSTA1 and GSTA2 expression. Pharmacogenetics 2001; 11: 663–669.
Di Pietro G, Magno LA, Rios-Santos F . Glutathione S-transferases: an overview in cancer research. Expert Opin Drug Metab Toxicol 2010; 6: 153–170.
Ning B, Wang C, Morel F, Nowell S, Ratnasinghe DL, Carter W et al. Human glutathione S-transferase A2 polymorphisms: variant expression, distribution in prostate cancer cases/controls and a novel form. Pharmacogenetics 2004; 14: 35–44.
Reszka E, Jablonowski Z, Wieczorek E, Gromadzinska J, Sosnowski M, Wasowicz W . GSTP1 mRNA expression in human circulating blood leukocytes is associated with GSTP1 genetic polymorphism. Clin Biochem 2011; 44: 1153–1155.
Gaziev J, Nguyen L, Puozzo C, Mozzi AF, Casella M, Perrone DM et al. Novel pharmacokinetic behavior of intravenous busulfan in children with thalassemia undergoing hematopoietic stem cell transplantation: a prospective evaluation of pharmacokinetic and pharmacodynamic profile with therapeutic drug monitoring. Blood 2010; 115: 4597–4604.
Johnson L, Orchard PJ, Baker KS, Brundage R, Cao Q, Wang X et al. Glutathione S-transferase A1 genetic variants reduce busulfan clearance in children undergoing hematopoietic cell transplantation. J Clin Pharmacol 2008; 48: 1052–1062.
Kim I, Keam B, Lee KH, Kim JH, Oh SY, Ra EK et al. Glutathione S-transferase A1 polymorphisms and acute graft-vs.-host disease in HLA-matched sibling allogeneic hematopoietic stem cell transplantation. Clin Transplant 2007; 21: 207–213.
Kusama M, Kubota T, Matsukura Y, Matsuno K, Ogawa S, Kanda Y et al. Influence of glutathione S-transferase A1 polymorphism on the pharmacokinetics of busulfan. Clin Chim Acta 2006; 368: 93–98.
ten Brink MH, Wessels JA, den Hartigh J, van der Straaten T, von dem Borne PA, Guchelaar HJ et al. Effect of genetic polymorphisms in genes encoding GST isoenzymes on BU pharmacokinetics in adult patients undergoing hematopoietic SCT. Bone Marrow Transplant 2012; 47: 190–195.
Abbasi N, Vadnais B, Knutson JA, Blough DK, Kelly EJ, O’Donnell PV et al. Pharmacogenetics of intravenous and oral busulfan in hematopoietic cell transplant recipients. J Clin Pharmacol 2011; 51: 1429–1438.
Zwaveling J, Press RR, Bredius RG, van Derstraaten TR, den Hartigh J, Bartelink IH et al. Glutathione S-transferase polymorphisms are not associated with population pharmacokinetic parameters of busulfan in pediatric patients. Ther Drug Monit 2008; 30: 504–510.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
Rifai N, Sakamoto M, Lafi M, Guinan E . Measurement of plasma busulfan concentration by high-performance liquid chromatography with ultraviolet detection. Ther Drug Monit 1997; 19: 169–174.
Zhong S, Wyllie AH, Barnes D, Wolf CR, Spurr NK . Relationship between the GSTM1 genetic polymorphism and susceptibility to bladder, breast and colon cancer. Carcinogenesis 1993; 14: 1821–1824.
Stephens M, Smith NJ, Donnelly P . A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 2001; 68: 978–989.
Ansari YTM, Rezgui S, Mezziani C, Desjean M, Vachon M et alBittencourt on behalf of the Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Association between busulfan exposure and outcome in children receiving intravenous busulfan before haematopoietic stem cell transplantation. Bone Marrow Transplant 2012; 47 Suppl: S40 (abstract 245).
Kim SD, Lee JH, Hur EH, Kim DY, Lim SN, Choi Y et al. Influence of GST gene polymorphisms on the clearance of intravenous busulfan in adult patients undergoing hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011; 17: 1222–1230.
Miyagi SJ, Brown IW, Chock JM, Collier AC . Developmental changes in hepatic antioxidant capacity are age-and sex-dependent. J Pharmacol Sci 2009; 111: 440–445.
Srivastava A, Poonkuzhali B, Shaji RV, George B, Mathews V, Chandy M et al. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood 2004; 104: 1574–1577.
Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant 1996; 17: 225–230.
Cantoni N, Gerull S, Heim D, Halter J, Bucher C, Buser A et al. Order of application and liver toxicity in patients given BU and CY containing conditioning regimens for allogeneic hematopoietic SCT. Bone Marrow Transplant 2011; 46: 344–349.
Kerbauy FR, Tirapelli B, Akabane H, Oliveira JS . The effect of administration order of BU and CY on toxicity in hematopoietic SCT in humans. Bone Marrow Transplant 2009; 43: 883–885.
Acknowledgements
We are thankful to all patients and their parents for consenting to participate in this study. This investigation was supported by grants provided by the Geneva Cancer League, CANSEARCH, Hans Wilsdorf, Telemaque and Charles Bruneau foundations. We thank the Swiss Oncology Group as our sponsor and the European Blood and Marrow Transplantation Pediatric (EBMT) working disease group for their support and for labeling this study as an EBMT trial. This study has been fully registered in a public trials registry as part of an ongoing prospective EBMT multicentric study (register at Clinical Trials.gov, NCT01257854). It is submitted on behalf of the Pediatric Disease Working Parties of the European Blood and Marrow Transplant group and is an EBMT label study (EudraCT number: 2009-018105-41).
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Ansari, M., Rezgui, M., Théoret, Y. et al. Glutathione S-transferase gene variations influence BU pharmacokinetics and outcome of hematopoietic SCT in pediatric patients. Bone Marrow Transplant 48, 939–946 (2013). https://doi.org/10.1038/bmt.2012.265
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.265
Keywords
This article is cited by
-
Prognostic factors for intermediate- or high-risk neuroblastomas in children in China
BMC Pediatrics (2023)
-
Effect of pharmacokinetics and pharmacogenomics in adults with allogeneic hematopoietic cell transplantation conditioned with Busulfan
Bone Marrow Transplantation (2023)
-
Role of Genetic Polymorphisms in Drug-Metabolizing Enzyme-Mediated Toxicity and Pharmacokinetic Resistance to Anti-Cancer Agents: A Review on the Pharmacogenomics Aspect
Clinical Pharmacokinetics (2022)
-
The analysis of GSTA1 promoter genetic and functional diversity of human populations
Scientific Reports (2021)
-
Review of the Pharmacokinetics and Pharmacodynamics of Intravenous Busulfan in Paediatric Patients
Clinical Pharmacokinetics (2021)