Abstract
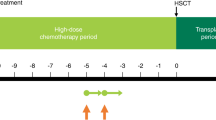
5-day/5-drug (5D/5D) is a novel high-dose regimen administered with autologous hematopoietic SCT (HSCT). It was designed to maximize cytoreduction via high dosing of synergistically interacting agents, while minimizing morbidity in patients with resistant neuroblastoma (NB) and ineligible for clinical trials due to myelosuppression from previous therapy. 5D/5D comprises carboplatin 500 mg/m2/day on days 1–2, irinotecan 50 mg/m2/day on days 1–3, temozolomide 250 mg/m2/day on days 1–3, etoposide 200 mg/m2/day on days 3–5 and cyclophosphamide 70 mg/kg/day on days 4–5. HSCT is on day 8. Sixteen patients received 21 courses. Treatment was in the outpatient clinic. Responses were noted against progressive disease (PD) that had developed while patients were off, or receiving only low-dose, chemotherapy but not against PD that emerged despite high-dose chemotherapy. Responses were also seen in patients with PD or stable disease after 131I-metaiodobenzylguanidine therapy. Grade 3 toxicities were limited to transient elevations in liver enzymes (three courses) and hyponatremia (one course). Bacteremia occurred in 2/21 (10%) courses. Hematological recovery allowed patients to be enrolled on clinical trials. In conclusion, 5D/5D (including HSCT) spares vital organs, entails modest morbidity, shows activity against resistant NB and helps patients meet eligibility requirements for formal clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Park JR, Scott JR, Stewart CF, London WB, Naranjo A, Santana VM et al. Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high risk neuroblastoma: a Children’s Oncology Group study. J Clin Oncol 2011, 4351–4357.
Saylors RL, Stine KC, Sullivan J, Kepner JL, Wall DA, Bernstein ML et al. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a Pediatric Oncology Group phase II study. J Clin Oncol 2001; 19: 3463–3469.
Kushner BH, Kramer K, Modak S, Cheung N-KV . Irinotecan plus temozolomide for relapsed or refractory neuroblastoma. J Clin Oncol 2006; 24: 5271–5276.
Wagner LM, Villablanca JG, Stewart CF, Crews KR, Groshen S, Reynolds CP et al. Phase I trial of oral irinotecan and temozolomide for children with relapsed high-risk neuroblastoma: a new approach to Neuroblastoma Therapy Consortium Study. J Clin Oncol 2009; 27: 1290–1296.
Garaventa A, Luksch R, Biasotti S, Severi G, Pizzitola MR, Viscardi E et al. A phase II study of topotecan with vincristine and doxorubicin in children with recurrent/refractory neuroblastoma. Cancer 2003; 98: 2488–2494.
Simon T, Längler A, Berthold F, Klingebiel T, Hero B . Topotecan and etoposide in the treatment of relapsed high-risk neuroblastoma: results of a phase-II trial. J Pediatr Hematol Oncol 2007; 29: 101–106.
Simon T, Längler A, Harnischmacher U, Frühwald MC, Jorch N, Claviez A et al. Topotecan, cyclophosphamide, and etoposide (TCE) in the treatment of high-risk neuroblastoma: results of a phase-II trial. J Cancer Res Clin Oncol 2007; 133: 653–661.
Kushner BH, Kramer K, LaQuaglia MP, Modak S, Yataghene K, Cheung N-KV . Reduction from seven to five cycles of intensive induction chemotherapy in children with high-risk neuroblastoma. J Clin Oncol 2004; 22: 4888–4892.
Kushner BH, Kramer K, Modak S, Yataghene K, Cheung N-KV . High-dose cyclophosphamide-irinotecan-vincristine for primary refractory neuroblastoma. Eur J Cancer 2010; 47: 84–89.
Kushner BH, Kramer K, Modak S, Cheung N-KV . High-dose carboplatin-irinotecan-temozolomide: treatment option for neuroblastoma resistant to topotecan. Pediatr Blood Cancer 2011; 56: 403–408.
Kushner BH, Kramer K, Modak S, Qin L-X, Cheung N-KV . Differential impact of high-dose cyclophosphamide, topotecan, and vincristine in clinical subsets of patients with chemoresistant neuroblastoma. Cancer 2010; 116: 3054–3060.
Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 1993; 11: 1466–77.
Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neuro-Oncol 2010; 97: 409–418.
Hawkins DS, Felgenhauer J, Park J, Kreissman S, Thomson B, Douglas J et al. Peripheral blood stem cell support reduces the toxicity of intensive chemotherapy for children and adolescents with metastatic sarcomas. Cancer 2002; 95: 1354–1365.
Pradhan KR, Johnson CS, Vik TA, Sender LS, Kreissman SG . A novel induction therapy for high-risk neuroblastoma utilizing sequential peripheral blood stem cell collection and infusion as hematopoietic support. Pediatr Blood Cancer 2006; 46: 793–802.
Grupp SA, Cohn SL, Wall D, Reynolds CP . Collection, storage, and infusion of stem cells in children with high-risk neuroblastoma: saving for a rainy day. Pediatr Blood Cancer 2006; 46: 719–722.
Matthay KK, Quach A, Huberty J, Franc BL, Hawkins RA, Jackson H et al. Iodine-131-metaiodobenzylguanidine double infusion with autologous stem-cell rescue for neuroblastoma: a new approach to Neuroblastoma Therapy phase I study. J Clin Oncol 2009; 27: 1020–1025.
Kreissman SG, Rackoff W, Lee M, Breitfeld PP . High dose cyclophosphamide with carboplatin: a tolerable regimen suitable for dose intensification in children with solid tumors. J Pediatr Hematol/Oncol 1997; 19: 309–312.
Bensimhon P, Villablanca JG, Sender LS, Matthay KK, Park JR, Seeger R et al. Peripheral blood stem cell support for multiple cycles of dose intensive induction therapy is feasible with little risk of tumor contamination in advanced stage neuroblastoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer 2010; 54: 596–602.
Strather D, Ashley D, Kellie SJ, Patel A, Jones-Wallace D, Thompson S et al. Feasibility of four consecutive high-dose chemotherapy cycles with stem-cell rescue for patients with newly diagnosed medulloblastoma or supratentorial primitive neuroectodermal tumor after craniospinal radiotherapy: results of a collaborative study. J Clin Oncol 2001; 19: 2696–2704.
Acknowledgements
Supported, in part, by grants from the National Institutes of Health (CA10450), Bethesda, MD; the Robert Steel Foundation, New York, NY; and Katie’s Find A Cure Fund, New York, NY.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kushner, B., Modak, S., Kramer, K. et al. 5-day/5-drug myeloablative outpatient regimen for resistant neuroblastoma. Bone Marrow Transplant 48, 642–645 (2013). https://doi.org/10.1038/bmt.2012.202
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.202