Abstract
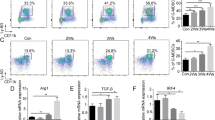
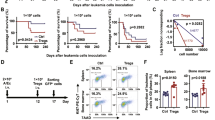
As a member of the B7 family, inducible co-stimulator ligand (ICOSLG) expressed on tumor cell has been reported to have an important role in tumor immunity. In this study, we sought to determine whether the expression of ICOSLG in mouse hematological malignancy cells influences GVL reaction after mouse allogeneic BMT. In our study, we analyzed the expression of ICOSLG in six mice hematological malignancy cell lines for the first time, and found that FBL3, A20 and P388 cells expressed high levels of ICOSLG. Then, we chose A20 cells as targets to construct a GVL model and study the effects on the GVL reaction by silencing the ICOSLG gene. The survival was analyzed. We found that in GVL model, mortality of interference groups was significantly delayed compared with the control group (P=0.0005). Our results indicate that knockdown of ICOSLG of mouse leukemic cells may significantly enhance GVL effect after allogeneic BMT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Her M, Kim D, Oh M, Jeong H, Choi I . Increased expression of soluble inducible costimulator ligand (ICOSL) in patients with systemic lupus erythematosus. Lupus 2009; 18: 501–507.
Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 1999; 397: 263–266.
Ling V, Wu PW, Finnerty HF, Bean KM, Spaulding V, Fouser LA, Leonard JP et al. Cutting edge: identification of GL50, a novel B7-like protein that functionally binds to ICOS receptor. J. Immunol 2000; 164: 1653–1657.
Freedman AS, Freeman GJ, Rhynhart K, Nadler LM . Selective induction of B7/BB-1 on interferon-gamma stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell Immunol 1991; 137: 429–437.
Linsley PS, Clark EA, Ledbetter JA . T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci USA 1990; 87: 5031–5035.
Yokochi T, Holly RD, Clark EA . B lymphoblast antigen (BB-1) expressed on epstein-barr virus-activated B cell blasts, B lymphoblastoid cell lines, and burkitt’s lymphomas. J Immunol 1982; 128: 823–827.
Bretscher P, Cohn M . A theory of self-nonself discrimination. Science 1970; 169: 1042–1049.
Subudhi SK, Zhou P, Yerian LM, Chin RK, Lo JC, Anders RA et al. Local expression of B7-H1 promotes organ-specific autoimmunity and transplant rejection. J Clin Invest 2004; 113: 694–700.
Swallow MM, Wallin JJ, Sha WC . B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity 1999; 11: 423–432.
Chen XL, Cao XD, Kang AJ, Wang KM, Su BS, Wang YL . In situ expression and significance of B7 costimulatory molecules within tissues of human gastric carcinoma. World J Gastroenterol 2003; 9: 1370–1373.
Schreiner B, Wischhusen J, Mitsdoerffer M, Schneider D, Bornemann A, Melms A et al. Expression of the B7-related molecule ICOSL by human glioma cells in vitro and in vivo. Glia 2003; 44: 296–301.
Wang S, Zhu G, Chapoval AI, Dong H, Tamada K, Ni J et al. Costimulation of T cells by B7-H2, a B7-like molecule that binds ICOS. Blood 2000; 96: 2808–2813.
Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature 1999; 402: 827–832.
Liang L, Sha WC . The right place at the right time: novel B7 family members regulate effector T cell responses. Curr Opin Immunol 2002; 14: 384–390.
Flies DB, Chen L . Modulation of immune response by B7 family molecules in tumor microenvironments. Immunol Invest 2006; 35: 395–418.
Flies DB, Chen L . The new B7s: playing a pivotal role in tumor immunity. J Immunother 2007; 30: 251–260.
Liu X, Bai XF, Wen J, Gao JX, Liu J, Lu P et al. B7H costimulates clonal expansion of, and cognate destruction of tumor cells by, CD8 (+) T lymphocytes in vivo. J Exp Med 2001; 194: 1339–1348.
Wallin JJ, Liang L, Bakardjiev A, Sha WC . Enhancement of CD8+ T cell responses by ICOS/B7h costimulation. J Immunol 2001; 167: 132–139.
Tamura H, Dan K, Tamada K, Nakamura K, Shioi Y, Hyodo H et al. Expression of functional B7-H2 and B7.2 costimulatory molecules and their prognostic implications in de novo acute myeloid leukemia. Clin Cancer Res 2005; 11: 5708–5717.
Yamashita T, Tamura H, Satoh C, Shinya E, Takahashi H, Chen L et al. Functional B7.2 and B7-H2 molecules on myeloma cells are associated with a growth advantage. Clin Cancer Res 2009; 15: 770–777.
Vieira PL, Wassink L, Smith LM, Nam S, Kingsbury GA, Gutierrez-Ramos JC et al. ICOS-mediated signaling regulates cytokine production by human T cells and provides a unique signal to selectively control the clonal expansion of Th2 helper cells. Eur J Immunol 2004; 34: 1282–1290.
Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity 2000; 13: 95–105.
Wang F, Zhu W, Liu T, Sun Z, Ju S, Ju S et al. The expression analysis of ICOS-L on activated T cells and immature dendritic cells as well as malignant B cells and Grave’s-disease-derived thyroid tissues by two novel mAbs against human ICOS-L. Tissue Antigens 2007; 69: 62–72.
Wang B, Cheng H, Wang L, Zhou H, Wang J . Expression of ICOSLG on mouse hematologic neoplasm cell lines and their influence on cytotoxicity in allogeneic mixed lymphocyte reactions. Leuk Lymphoma 2012; 53: 674–680.
Chen BJ, Cui X, Liu C, Chao NJ . Prevention of graft-versus-host disease while preserving graft-versus-leukemia effect after selective depletion of host-reactive T cells by photodynamic cell purging process. Blood 2002; 99: 3083–3088.
Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, Sharpe AH, Noelle RJ, Rudensky AY et al. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM). Blood 2005; 105: 3372–3380.
Hubbard VM, Eng JM, Ramirez-Montagut T, Tjoe KH, Muriglan SJ, Kochman AA et al. Absence of inducible costimulator on alloreactive T cells reduces graft versus host disease and induces Th2 deviation. Blood 2005; 106: 3285–3292.
Ara G, Baher A, Storm N, Horan T, Baikalov C, Brisan E et al. Potent activity of soluble B7RP-1-Fc in therapy of murine tumors in syngeneic hosts. Int J Cancer 2003; 103: 501–507.
Tao Y, Zhang W, Fang Y, Yang D, Wang L, Zhou H et al. Bortezomib attenuates acute graft-vs.-host disease through interfering with host immature dendritic cells. Exp Hematol 2011; 39: 710–720.
Bektas M, Payne SG, Liu H, Goparaju S, Milstien S, Spiegel S . A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J Cell Biol 2005; 169: 801–811.
Yu XZ, Liang Y, Nurieva RI, Guo F, Anasetti C, Dong C . Opposing effects of ICOS on graft-versus-host disease mediated by CD4 and CD8 T cells. J Immunol 2006; 176: 7394–7401.
Strauss L, Bergmann C, Szczepanski MJ, Lang S, Kirkwood JM, Whiteside TL . Expression of ICOS on human melanoma-infiltrating CD4+CD25highFoxp3+ T regulatory cells: implications and impact on tumor-mediated immune suppression. J Immunol 2008; 180: 2967–2980.
Frauwirth KA, Thompson CB . Activation and inhibition of lymphocytes by costimulation. J Clin Invest 2002; 109: 295–299.
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (NSFC No. 30871100, 81090110) to JMW, NSFC No. C140402 to HYQ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Wang, B., Ma, N., Cheng, H. et al. Effects of ICOSLG expressed in mouse hematological neoplasm cell lines in the GVL reaction. Bone Marrow Transplant 48, 124–128 (2013). https://doi.org/10.1038/bmt.2012.103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.103