Abstract
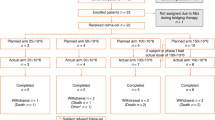
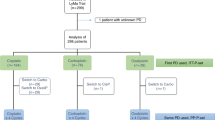
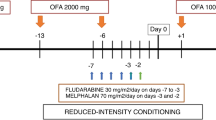
We retrospectively analyzed 44 patients undergoing first-line treatment for mantle cell lymphoma with R-HyperCVAD, with or without rituximab (R) maintenance or auto-SCT. The primary study end point was PFS; secondary end point was overall survival.
Median follow up for all patients was 3.3 years. Median age was 54 years, and 95% (n=42) were stage III or IV at diagnosis. In all, 17 patients underwent consolidative auto-SCT and 12 patients received R maintenance. The overall response rate was 95%, with 91% achieving complete response (CR). Median PFS for all patients was 3.5 years. Median PFS was 2.3 years for patients treated with R-HyperCVAD alone vs 3.9 years (P=0.02) with R-HyperCVAD+ R maintenance and 4.5 years (P=0.01) with R-HyperCVAD+ auto-SCT. For patients who did not achieve CR at interim staging, PFS for R-HyperCVAD alone was 1.4 years vs not reached for R-HyperCVAD+ consolidation (either R maintenance or auto-SCT) (P=0.02). PFS for patients with CR at interim staging was 3.3 years vs not reached (P=0.04) after consolidation. Our data suggest potential improvement in PFS when R-HyperCVAD is consolidated with either R maintenance or auto-SCT. This benefit appears particularly significant in those patients who do not achieve CR at interim restaging.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Armitage JO, Weisenburger DD . New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol 1998; 16: 2780–2795.
Zucca E, Roggero E, Pinotti G, Pedrinis E, Cappella C, Venco A et al. Patterns of survival in mantle cell lymphoma. Ann Oncol 1995; 6: 257–262.
Howard OM, Gribben JG, Neuberg DS, Grossbard M, Poor C, Janicek MJ et al. Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: molecular complete responses are not predictive of progression-free survival. J Clin Oncol 2002; 20: 1288–1294.
Lenz G, Dreyling M, Hoster E, Wormann B, Duhrsen U, Metzner B et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol 2005; 23: 1984–1992.
Gianni AM, Magni M, Martelli M, Di Nicola M, Carlo-Stella C, Pilotti S et al. Long-term remission in mantle cell lymphoma following high-dose sequential chemotherapy and in vivo rituximab-purged stem cell autografting (R-HDS regimen). Blood 2003; 102: 749–755.
Khouri IF, Saliba RM, Okoroji GJ, Acholonu SA, Champlin RE . Long-term follow-up of autologous stem cell transplantation in patients with diffuse mantle cell lymphoma in first disease remission: the prognostic value of beta2-microglobulin and the tumor score. Cancer 2003; 98: 2630–2635.
Vandenberghe E, Ruiz de Elvira C, Loberiza FR, Conde E, Lopez-Guillermo A, Gisselbrecht C et al. Outcome of autologous transplantation for mantle cell lymphoma: a study by the European Blood and Bone Marrow Transplant and Autologous Blood and Marrow Transplant Registries. Br J Haematol 2003; 120: 793–800.
Dreyling M, Lenz G, Hoster E, Van Hoof A, Gisselbrecht C, Schmits R et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood 2005; 105: 2677–2684.
Lefrere F, Delmer A, Levy V, Delarue R, Varet B, Hermine O . Sequential chemotherapy regimens followed by high-dose therapy with stem cell transplantation in mantle cell lymphoma: an update of a prospective study. Haematologica 2004; 89: 1275–1276.
Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol 2005; 23: 7013–7023.
Romaguera JE, Fayad LE, Feng L, Hartig K, Weaver P, Rodriguez MA et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol 2010; 150: 200–208.
Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood 2008; 112: 2687–2693.
Hochster H, Weller E, Gascoyne RD, Habermann TM, Gordon LI, Ryan T et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 Study. J Clin Oncol 2009; 27: 1607–1614.
van Oers MH, Klasa R, Marcus RE, Wolf M, Kimby E, Gascoyne RD et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood 2006; 108: 3295–3301.
Kahl BS, Longo WL, Eickhoff JC, Zehnder J, Jones C, Blank J et al. Maintenance rituximab following induction chemoimmunotherapy may prolong progression-free survival in mantle cell lymphoma: a pilot study from the Wisconsin Oncology Network. Ann Oncol 2006; 17: 1418–1423.
Forstpointner R, Unterhalt M, Dreyling M, Bock HP, Repp R, Wandt H et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood 2006; 108: 4003–4008.
Herrmann A, Hoster E, Zwingers T, Brittinger G, Engelhard M, Meusers P et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol 2009; 27: 511–518.
Hainsworth JD, Litchy S, Burris III HA, Scullin Jr DC, Corso SW, Yardley DA et al. Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin's lymphoma. J Clin Oncol 2002; 20: 4261–4267.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–586.
GhieDmini M, Schmitz SF, Cogliatti S, Bertoni F, Waltzer U, Fey MF et al. Effect of single-agent rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: a study of the Swiss Group for Clinical Cancer Research (SAKK). J Clin Oncol 2005; 23: 705–711.
Acknowledgements
TA is a Special Fellow in Clinical Research of the Leukemia and Lymphoma Society
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ahmadi, T., McQuade, J., Porter, D. et al. Potential prolongation of PFS in mantle cell lymphoma after R-HyperCVAD: auto-SCT consolidation or rituximab maintenance. Bone Marrow Transplant 47, 1082–1086 (2012). https://doi.org/10.1038/bmt.2011.218
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2011.218
Keywords
This article is cited by
-
Maintenance Therapy in Diffuse Large B Cell Lymphoma and Mantle Cell Lymphoma
Current Treatment Options in Oncology (2018)
-
Multiple infusions of CD20-targeted T cells and low-dose IL-2 after SCT for high-risk non-Hodgkin's lymphoma: A pilot study
Bone Marrow Transplantation (2014)