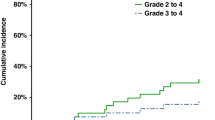
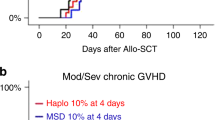
Abstract
Cutaneous T-cell lymphomas (CTCL) are a heterogeneous group of non-Hodgkin lymphomas that are considered incurable. The role of allogeneic hematopoietic SCT (HSCT) in the treatment of CTCL is not well defined but may provide potent graft-vs-lymphoma (GVL) activity independent of the conditioning therapy. We present outcomes of 12 extensively-pretreated patients with CTCL who underwent allogeneic HSCT using, most commonly, a reduced intensity conditioning regimen. Median age at diagnosis of CTCL was 49 years, and median time to transplantation from diagnosis was 3.3 years. Transplantation induced and maintained CR in six patients with active disease, supporting the presence of a GVL effect. TRM was low, and 42% of patients were alive and disease-free a median duration of 22 months after transplant. Two patients showed strong and direct evidence of a GVL-effect with a direct response to withdrawal of immunosuppression or to donor leukocyte infusion. Our data show that HSCT can provide long-term disease control in patients with advanced CTCL, which otherwise was refractory to immunotherapy and chemotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
van Doorn R, Van Haselen CW, van Voorst Vader PC, Geerts M-L, Heule F, de Rie M et al. Mycosis fungoides: disease evolution and prognosis of 309 Dutch patients. Arch Dermatol 2000; 136: 504–510.
Bernengo MG, Quaglino P, Novelli M, Cappello N, Doveil GC, Lisa F et al. Prognostic factors in Sézary syndrome: a multivariate analysis of clinical, haematological and immunological features. Ann Oncol 1998; 9: 857–863.
Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood 2007; 109: 31–39.
Zain J, O’Connor OA . Targeting histone deacetyalses in the treatment of B- and T-cell malignancies. Invest New Drugs 2010; 28: S58–S78.
Erter J, Alinari L, Darabi K, Gurcan M, Garzon R, Marcucci G et al. New targets of therapy in T-cell lymphomas. Curr Drug Targets 2010; 11: 482–493.
Grant C, Rahman F, Piekarz R, Peer C, Frye R, Robey RW et al. Romidepsin: a new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Expert Rev Anticancer Ther 2010; 10: 997–1008.
Whittaker SJ, Demierre MF, Kim EJ, Rook AH, Lerner A, Duvic M et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol 2010; 28: 4485–4491.
Rook AH, Kuzel TM, Olsen EA . Cytokine therapy of cutaneous T-cell lymphoma: interferons, interleukin-12, and interleukin-2. Hematol Oncol Clin North Am 2003; 17: 1435–1448.
Choi J, Foss F . Cutaneous T-cell lymphoma: biologic targets for therapy. Curr Hematol Malig Rep 2007; 2: 272–277.
Horwitz SM . Novel therapies for cutaneous T-cell lymphomas. Clin Lymphoma Myeloma 2008; 8: S187–S192.
Bigler RD, Crilley P, Micaily B, Brady LW, Topolsky D, Bulova S, et al. Autologous bone marrow transplantation for advanced stage mycosis fungoides. Bone Marrow Transplant 1991; 7: 133–137.
Goldstein SC, Porter DL . Allogeneic immunotherapy to optimize the graft-versus-tumor effect: concepts and controversies. Expert Rev Hematol 2010; 3: 301–314.
Molina A, Zain J, Arber DA, Angelopolou M, O'Donnell M, Murata-Collins J et al. Durable clinical, cytogenetic, and molecular remissions after allogeneic hematopoietic cell transplantation for refractory Sezary syndrome and mycosis fungoides. J Clin Oncol 2005; 23: 6163–6171.
Duvic M, Donato M, Dabaja B, Richmond H, Singh L, Wei W et al. Total skin electron beam and non-myeloablative allogeneic hematopoietic stem-cell transplantation in advanced mycosis fungoides and Sezary syndrome. J Clin Oncol 2010; 28: 2365–2372.
Delioukina M, Zain J, Palmer JM, Tsai N, Thomas S, Forman S . Reduced-intensity allogeneic hematopoietic cell transplantation using fludarabine-melphalan conditioning for treatment of mature T-cell lymphomas. Bone Marrow Transplant 2012; 47: 65–72.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Amer Statist Assoc 1958; 53: 457–481.
Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007; 110: 1713–1722.
Lansigan F, Foss FM . Current and emerging treatment strategies for cutaneous T-cell lymphoma. Drugs 2010; 70: 273–286.
Gardner JM, Evans KG, Musiek A, Rook AH, Kim EJ . Update on treatment of cutaneous T-cell lymphoma. Curr Opin Oncol 2009; 21: 131–137.
Prince HM, Duvic M, Martin A, Sterry W, Assaf C, Sun Y et al. Phase III placebo-controlled trial of denileukin diftitox for patients with cutaneous T-cell lymphoma. J Clin Oncol 2010; 28: 1870–1877.
Duarte RF, Canals C, Onida F, Gabriel IH, Arranz R, Arcese W et al. Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sézary syndrome: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 2010; 28: 4492–4499.
Acknowledgements
This work was supported in part by grants from The Leukemia & Lymphoma Society (7000-02) and NIH (K24 CA11787901) (DLP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Alain H Rook is on the Speaker's bureau of Therakos, and is a consultant for HY Biopharma. Ellen J Kim has obtained research funding from TenX, Biocryst, Genmab, Glouchester, Celgene, and is a consultant for Eisai. Other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Paralkar, V., Nasta, S., Morrissey, K. et al. Allogeneic hematopoietic SCT for primary cutaneous T cell lymphomas. Bone Marrow Transplant 47, 940–945 (2012). https://doi.org/10.1038/bmt.2011.201
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2011.201
Keywords
This article is cited by
-
Long term outcomes of nonmyeloablative allogeneic stem cell transplantation with TSEB TLI and ATG for Mycosis Fungoides and Sezary Syndrome
Bone Marrow Transplantation (2024)
-
Successful salvage therapy for refractory primary cutaneous gamma-delta T-cell lymphoma with a combination of brentuximab vedotin and gemcitabine
Experimental Hematology & Oncology (2021)
-
Allogeneic hematopoietic stem cell transplantation in Primary Cutaneous T Cell Lymphoma
Annals of Hematology (2018)
-
Multidisciplinary Management of Mycosis Fungoides/Sézary Syndrome
Current Hematologic Malignancy Reports (2017)