Abstract
Diagnosis of acute intestinal GVHD (aGVHD) following allogeneic hematopoietic cell transplantation is based on clinical symptoms and histological lesions. This retrospective analysis aimed to validate the ‘Freiburg Criteria’ for the endoscopic grading of intestinal aGVHD. Grade 1: no clear-cut criteria; grade 2: spotted erythema; grade 3: aphthous lesions; and grade 4: confluent defects, ulcers, denudation of the mucosa. Having excluded patients with infectious diarrhea, we evaluated 175 consecutive patients between January 2001 and June 2009. Setting a cutoff between grade 1 (no change in therapy) and grade 2 (intensification of immunosuppression), macroscopy had a sensitivity of 89.2% (95% confidence interval (CI): 80.4–94.9%), a specificity of 79.4% (95% CI: 69.6–87.1%), a positive-predictive value of 79.6% (95% CI: 70.0–87.2%) and a negative-predictive value of 89.0% (95% CI: 80.2–94.9%). In all, 20% of patients with aGVHD in the lower gastrointestinal tract (GIT) had lesions only in the terminal ileum. In all patients with aGVHD ⩾2 of the upper GIT, typical lesions were also found in the lower GIT. Ileo-colonoscopy showed the highest diagnostic yield for aGVHD. In conclusion, the ‘Freiburg Criteria’ for macroscopic diagnosis of intestinal aGVHD provide high accuracy for identifying aGVHD ⩾2.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic SCT has become an essential part of the standard therapeutic repertoire in several hematological diseases, such as acute or chronic leukemias, other hematological malignancies, inborn metabolic errors and conditions of BM failure.1, 2, 3, 4, 5, 6, 7 Frequent complications are infections, side effects from the conditioning regime or medications, and GVHD with skin, gut or bile ducts, as its the main organ manifestations. For historical rather than clinical or pathophysiological reasons, GVHD has been categorized in acute GVHD, arising before day +100 after transplantation, and chronic GVHD, arising later than day +100.8, 9, 10, 11 However, this strict differentiation has been abandoned. A condition which arises after day +100 after SCT and shows the clinical features of the classical acute intestinal GVHD (aGVHD) is regarded as a late-onset aGVHD.12, 13, 14 Acute GVHD affects mainly the skin, liver and gastrointestinal tract (GIT).15, 16, 17, 18 Leading symptoms are rash or erythema, jaundice, diarrhea and nausea. In late chronic GVHD, the symptoms gradually evolve into a scleroderma-like syndrome.19, 20, 21, 22 Gold standard for diagnosis is histology (skin, liver, gut), and there are long-established criteria for its histological diagnosis and grading. The most important signs of intestinal aGVHD are crypt cell apoptosis and the presence of crypt loss.12, 23, 24 However, before day +20 after SCT, similar lesions can also be caused by the conditioning regime itself.24 The grading of intestinal aGVHD has been suggested to fall between 1 and 4 according to the degree of histological characteristics. It is generally accepted that for a correct diagnosis of intestinal GVHD, biopsies from the GIT must be obtained. Clinical experience has shown that histologically proven intestinal aGVHD ⩾2 reflects clinically evident intestinal aGVHD ⩾2 very well, and is therefore a clear indication for intensifying immunosuppressive therapy.25
Diarrhea after SCT is an ambiguous symptom. Its main causes are drug-induced side effects, chemotherapy or radiation-induced toxicity, infections and GVHD.26, 27 Microbiological investigation is the first step in the diagnostic work-up in such patients. The presence of thrombocytopenia often prevents endoscopy with biopsies, and hematologists were long reluctant to do colonoscopies in these patients because of worries about complications associated with invasive procedures and the ambiguous diagnostic yield. Our paper published in 1994 was the first systematic survey to evaluate the macroscopic findings obtained during colonoscopy and compare them with histology. Our criteria for the endoscopic diagnosis of aGVHD of the lower GIT compared well with histological findings.28 Later on, other authors used slightly different endoscopic criteria for diagnosing intestinal aGVHD grades 1–4. However, these studies could not establish such a simple stepwise grading system from macroscopic findings (grades 1–4), which readily parallel with histological grades (1–4).14, 29, 30, 31, 32, 33 Therefore, these criteria cannot be easily used for immediately predicting whether or not an aGVHD grade ⩾2 exists. Thompson et al.31 used our grading system in a relatively small cohort of 24 patients, finding some correlation between histological and clinical data, but their data were insufficient to guide the clinician to a therapeutic decision.
In this paper, we present a retrospective analysis of our endoscopic data, in which to our knowledge is the largest cohort of patients after Allo-SCT. From a clinical point of view, it is sufficient to define whether an aGVHD ⩾2 exists, because this diagnosis requires intensive immunosuppression. Accordingly, we have slightly modified our original criteria: we have now decided against defining grade 1 criteria, because this diagnosis is doubtful and does not lead to a change in therapy. The primary aim of this study was to investigate whether diagnosis of histological aGVHD ⩾2 can be based on the macroscopic findings in the colon and terminal ileum confirming the validity of the endoscopic ‘Freiburg Criteria’. Therefore, we compared them with histology, which is and remains the gold standard for diagnosis. In addition, we evaluated the distribution of aGVHD lesions along the GIT in order to get information about the best endoscopic diagnostic approach.
Patients and methods
Patients
Allogeneic hematopoietic cell transplantation was performed in 751 patients in our department between January 2001 and June 2009. Colonoscopy was performed in 215 patients because of suspected aGVHD. The indication for endoscopy was diarrhea, nausea, vomiting or other GIT symptoms. Microbiological tests were performed immediately if aGVHD was suspected, endoscopies were performed one (or two) day(s) later. Patients are prepared for colonoscopy with a PEG-containing solution. In all, 175 consecutive patients could be included into this study, who had both endoscopic and histological findings available, were at least 20 days post allogeneic hematopoietic cell transplantation and did not have an infectious process identified. In all, 56% of the 175 patients were male, 44% were female. Mean age was 53 years, s.d. 13 years. The underlying diseases were AML (43%), myelodysplastic syndrome (MDS) (14%), ALL (10%), non-Hodgkin lymphoma (NHL) (10%), CLL (9%), CML (4%), other myeloproliferative syndrome (MPS) (4%), malignant melanoma (MM) (3%) and others (3%).
The conditioning regimes were combinations consisting of fludarabine, BU, cyclophosphamid, BCNU (carmustine), melphalan, thiotepa, treosulfan, etoposide (VP16) or TBI. For prophylaxis of aGVHD the following drugs were used: CsA, alemtuzumab, mycophenolate mofetil, MTX and ATG.
Endoscopy
We compared the macroscopic and histological findings in 175 patients in whom a concomitant intestinal infection had been excluded. If the same patient had had several colonoscopies, we only evaluated the first one. During the first year of the study, we only aimed to perform an inspection of the total colon and we did an ileoscopy only in part of the cases. Only later, we perceived that in several patients typical aGVHD lesions can be found only in the terminal ileum. Therefore, the terminal ileum was intubated during the colonoscopy in only 74 patients. Forty-one patients underwent both gastroscopy and colonoscopy, and biopsies could be obtained in all of them. Colonoscopies and/or gastroscopies were performed by experienced endoscopists using Olympus endoscopes (CF 130 L, CF 140 I, CF Q 145 AI, CF-H 180 AI, GIF Q 145, GIF Q 165, GIF H 180, Olympus, Hamburg, Germany). All macroscopic findings were evaluated according to the ‘Freiburg Criteria’. The endoscopists recorded their interpretation immediately after the endoscopies. Later on, all pictures and interpretations were cross-checked by WK.
Biopsies were taken from different sites in the GIT: at least one biopsy was taken in the ascending, transverse and descending colon, and in the rectosigmoid. In 56 cases, biopsies were taken from the terminal ileum. Biopsies were stained using hematoxylin–eosin and evaluated using the latest criteria for histological diagnosis of aGVHD,12 which are indicated in Table 1 in the Results section. The presence of CMV infection was excluded by staining for CMV Ag. Histological specimens were mainly evaluated by ASG. All participating pathologists used the same criteria for histological grading.12
Macroscopic criteria for aGVHD (‘Freiburg Criteria’)
The criteria for diagnosing and grading intestinal aGVHD (‘Freiburg Criteria’) are indicated in Table 2. The differences to our original criteria from 1994 are slight.28 The macroscopic diagnosis of aGVHD grade 1 is ambiguous and the consequences of no aGVHD and aGVHD grade 1 are the same, namely, no change in therapy. We therefore focused on identifying aGVHD ⩾2, and described lesions typical for the grades 2–4. If there were no grade ⩾2 lesions, we state only that there is no aGVHD ⩾2. This should lead the clinician to decide against changing the actual therapy. If an aGVHD ⩾2 was diagnosed, it was an indicator to the clinician that immunosuppressive therapy should be intensified.
We draw attention to the fact that these criteria primarily refer to the lesions in the terminal ileum and colon only. Comparison between histology and macroscopy refers to terminal ileum and colon as well. However, for evaluating the diagnostic accuracy of upper and lower GIT endoscopy only histological findings were compared.
Results
Time interval after SCT
The time after SCT is shown in Figure 1. Endoscopies were performed between days +21 and +405. Endoscopies were performed before day +100 in 65%, after day +100 in 35%.
Histological and macroscopic grading of aGVHD
Histological grading of intestinal aGVHD
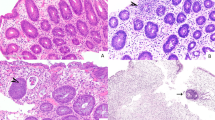
The criteria for the histological grading of intestinal aGVHD according to Washington et al.12 are indicated in Table 1. Figure 2 shows examples of the grades of aGVHD from grades 1 to 4.
Macroscopic grading of intestinal aGVHD
The criteria for grading gastrointestinal aGVHD macroscopically (‘Freiburg Criteria’) in the terminal ileum and the colon are indicated in Table 2. Figure 3 illustrates macroscopic examples of aGVHD from grades 2 to 4.
Comparison of macroscopy and microscopy
Table 3 contains a survey of the endoscopic and histological grading data and the comparison between macroscopic and histological grading of aGVHD in the terminal ileum and colon in the 175 ileo-colonoscopies.
Macroscopy and histology were graded identically in 71%, and in 18% there was a difference of ±1 grade. We observed a difference of more than 2 grades in only 11%.
Table 4 indicates sensitivities and specificities of the macroscopic criteria in comparison with histology, which is used as the gold standard. It indicates the calculation using a cutoff between grades 1 and 2 for macroscopy and histology. The cutoff value between histological grades 1 and 2 reflects the cutoff for the decision whether to start immunosuppressive therapy or not. Table 4 also explains the calculation using a cutoff between grades 2 and 3 for macroscopy, and between grades 1 and 2 for histology. In practice, macroscopic grade 3 aGVHD is easier to diagnose than grade 2. As expected, the cutoff between grades 1 and 2 yields high sensitivity and a little less specificity. When the cutoff is set between grades 2 and 3, a little sensitivity is lost but the specificity increases.
If we keep the cutoff between histology at grades 1 and grade 2, vary the cutoff for the macroscopy and calculate the ROC, the Youden index (sensitivity+specificity–1) is as follows: 0.685 for cutoff 1/2, 0.665 for cutoff 2/3, and 0.572 for cutoff 3/4. Therefore, in order to achieve a diagnostic endoscopic procedure with the highest sensitivity and specificity by setting the cutoff between grades 1 and 2 seems optimal for both histology and macroscopy.
Involvement of different parts of the GIT in aGVHD
Distribution of aGVHD in the GIT
As shown in Figure 4, the different grades of aGVHD are unevenly distributed in the GIT. Remarkably, the frequency of grade 4 aGVHD in the terminal ileum or duodenum was 54% and 33.3%, respectively, whereas grade 4 aGVHD occurred in only 18.4% of the total cohort of patients with colonoscopy (without or with ileoscopy). These data suggest that the small bowel shows grade 4 involvement more frequently than does the colon, and that the terminal ileum may be affected by this disease without the involvement of colon. However, it is important to remember that these data were collected in a selected subset of the 175 patients and that they do not necessarily reflect the true distribution.
Is ileoscopy necessary in colonoscopy?
The terminal ileum was inspected on colonoscopy in 74 patients, and we could evaluate histological grading in 56 patients with acute GVHD grade ⩾1. In all, 39 patients (69.9%) had aGVHD grade ⩾2. Overall, 13 had these lesions in the colon only, 18 in the colon and terminal ileum. In 8 patients (20%) with aGVHD ⩾2 the terminal ileum was involved exclusively and the colon was normal (Figure 5a). This demonstrates that the terminal ileum should be inspected whenever possible.
Diagnostic yield of histology of different parts of the gastrointestinal tract. (a) Only colonoscopy vs colonoscopy+ileoscopy. Number of patients with histologically proven aGVHD ⩾1 in: only colon; colon and terminal ileum; and only terminal ileum. Number of patients with histologically proven aGVHD ⩾2 in: only colon; colon and terminal ileum; only terminal ileum. (b) Ileo-colonoscopy vs upper GIT endoscopy vs combination of both. Number of patients with histologically proven aGVHD ⩾1 in: only lower GIT; lower and upper GIT; and only upper GIT. Number of patients with histologically proven aGVHD ⩾2 in: only lower GIT; lower and upper GIT; and only upper GIT. Term. ileum, terminal ileum.
Upper and/or lower GIT endoscopy
Endoscopies of the upper and lower GIT at the same time point were performed in 41 patients. Figure 5b shows that histological aGVHD ⩾2 lesions were never identified solely in the upper GIT and not in the lower GIT, when including the terminal ileum. These data suggest that a colonoscopy including inspection of the terminal ileum has the highest diagnostic yield, which is not increased by additional upper GIT endoscopy.
In a small subset of 15 out of these 41 patients, histology in rectum sigmoid colon, other parts of the colon, terminal ileum, stomach and duodenum could be evaluated at the same time (Table 5). In all 15 patients we found histological grade ⩾1 aGVHD in at least one region of the GIT and grade ⩾2 aGVHD in 12 of them likewise in at least one part of the GIT. Table 5 illustrates the diagnostic accuracy of histological evaluation of different parts of the GIT and combination of endoscopies if one evaluates these 15 patients. Inspection of rectum and sigmoid colon found aGVHD ⩾2 in 7/12 patients, inspection of the total colon yielded the correct diagnosis in 10/12 patients. The yield of colonoscopy with ileoscopy for diagnosing aGVHD ⩾2 amounts to 100% (12/12 patients). These data suggest that an ileoscopy detects aGVHD requiring therapy in several patients with normal findings in the colon. However, the combination of recto-sigmoidoscopy and esophago-gastro-duodenoscopy (including histology in the duodenum) may be equivalent. Alternatively: An esophago-gastro-duodenoscopy yielded no further information if ileo-colonoscopy has been done.
Discussion
To our knowledge this is by far the largest study addressing the diagnostic value of endoscopic and macroscopic diagnosis of intestinal aGVHD. We demonstrate that using the modified ‘Freiburg Criteria’ in ileo-colonoscopy, macroscopy has high sensitivity and specificity for diagnosing aGVHD ⩾2 and it can therefore guide the clinician to a rapid therapeutic decision. In this evaluation, we set a cutoff for macroscopy and histology between aGVHD grades 1 and 2, because it is generally accepted that a histologically diagnosed intestinal aGVHD ⩾2 is an indication for intensifying immunosuppressive therapy. In contrast to our earlier paper,28 we have refrained from defining the macroscopic criteria for aGVHD grade 1. The rationale behind that decision was that its potential signs are ambiguous, and the only clinical consequence is not to change the therapy. Therefore, only the differentiation between grades 0–1 (no change in therapy) and grade ⩾2 is clinically relevant. Beginning from grade 2 there is a stepwise progression of macroscopic lesions from spotted erythema (‘initial aphthous lesions’; grade 2) to ‘complete denudation of the mucosa’ (grade 4), which parallels the stepwise progression of the histological lesions. Our data thus suggest that applying the ‘Freiburg Criteria’ in ileo-colonoscopy could be an important tool for the clinician in making a therapeutic decision. The decision not to change therapy or to intensify immunosuppression can be made immediately using these macroscopic criteria, whereas histology would delay the change in therapy by at least 1 day.
Our study included patients with an endoscopy between days +20 and 405 after SCT. The classical differentiation between GVHD arising before day 100 (acute GVHD) and after day 100 (chronic GVHD) has meanwhile been abandoned.12, 13, 14 In particular, the use of less intensity-conditioning regimes led to a late occurrence of a clinical situation that is classified as ‘delayed acute GVHD’ with no difference to the ‘classical acute GVHD’. Alternatively, persistent acute GVHD is found after day +100. Endoscopic findings from patients were therefore included in our study if they presented with the classical clinical symptoms of acute GVHD (gut, liver, skin) without clinical manifestations suggesting cGVHD.
Several studies have evaluated the diagnostic yield of macroscopic aspects of endoscopy in comparison with histology. The first study was our own paper from 1994,28 in which we described the high sensitivity and specificity of macroscopy compared with histology in the recto-sigmoidoscopies of 42 patients after allogeneic BMT. In a cohort of 44 patients, Cruz-Correa et al.32 described slightly different criteria for macroscopic diagnosis. Combining findings in patients with all grades of aGVHD (grades 1–4) and combining their findings in the lower and upper GIT, they reported a sensitivity of 83% (10/12), a specificity of 69% (22/32), a positive-predictive value of 50% (10/20) and a negative-predictive value of 92% (22/24). However, because of the mixture of upper and lower GIT findings, it is difficult to translate their data into clear-cut therapeutic recommendations for the clinician. The authors reported no data about findings in the terminal ileum. Cheung et al.30 used the Cruz-Correa criteria, focussing on sigmoidoscopy and gastroscopy, and concluding that the overall reliability of the endoscopic diagnosis of aGVHD remains inadequate. Thompson et al.31 used the macroscopic criteria we described. However, they observed little concordance between macroscopic and histological findings (38.9%) with a difference of 2–4 grades between macroscopy and histology in 28.2%, focussing on the diagnostic yield of biopsies in different parts of the lower and upper GIT for diagnosing all grades of aGVHD. In a small cohort of 24 patients with suspected aGVHD, they found a diagnostic yield of histology in the distal colon of 82%, and nearly identical data for upper GIT endoscopy+sigmoidoscopy and colonoscopy+ileal biopsies (about 94%). There is an important difference between our study and the data of Thompson: Thompson et al. focussed on the presence of aGVHD of any grade (that is, grade 1–4) and they did not differentiate between grade 1—without clinical consequences—and higher grades—with clinical consequence of intensification of immunosuppression. They reported a high rate of aGVHD of 44.7% even in endoscopically normally appearing regions. This can be explained by the fact that there are no reliable macroscopic signs of grade 1 aGVHD (we neglected a possible grade 1 aGVHD as mentioned above!) and that macroscopic grade 4 aGVHD in the terminal ileum is difficult to discriminate from grade 0 to 1. Ross et al.14 reported a higher diagnostic accuracy of biopsies in the rectosigmoid colon than in the upper GIT. However, their data cannot be compared with ours, because the authors did not perform total ileo-colonoscopies and they did not differentiate between grade 1 aGVHD and grades 2–4. They performed esophago-gastro-duodenoscopy and recto-sigmoidoscopy. If histological criteria of aGVHD at least grade 1 were fulfilled, the patient was classified as having aGVHD. In this setting, recto-sigmoidoscopy had the highest sensitivity and specificity. On contrast, we did a complete colonoscopy and in part of the patients ileo-colonoscopy and we focus on patients with aGVHD ⩾2 (requiring start or intensification of therapy). In this setting, we found that in about 20% of the patients with ileo-colonoscopy typical aGVHD lesions could be found only in the terminal ileum that would have been missed if only recto-sigmoidoscopy was performed. Apart from that: it was not only a primary aim of our study to describe the occurrence of aGVHD along the GIT but also to evaluate macroscopic criteria that fit very well to the histological classification. However, the observation of an isolated manifestation of aGVHD in the terminal ileum is clinically relevant and should be evaluated in a prospective study.
The high diagnostic accuracy described in our study may be due to several reasons: (1) our group has over 15 years of experience with endoscopy in GVHD patients, and all of these endoscopies have been supervised or double-checked by the investigator with the greatest expertise (WK). (2) The evaluation of macroscopic results focuses on grade ⩾2 lesions which reveal alterations that can easily be diagnosed (grade 3 lesions are the most distinct lesions!). (3) Comparison between macroscopy and histology concerns only ileo-colonoscopic findings and not those of gastro-duodenoscopy, because experience has shown us that it is difficult to transfer our criteria to lesions found in the upper GIT. (4) It should be considered in all comparisons between macroscopy and histology that histological lesions in aGVHD of the stomach or even duodenum are less well defined than in the lower GIT.12, 14, 33, 34
An important aspect of endoscopy in aGVHD patients is the potential similarity between lesions in the GIT due to aGVHD and gastrointestinal infections.35, 36 For our final evaluation we eliminated 19 patients with CMV infection or cryptosporidia. CMV infection in particular may mimic all grades of aGVHD. However, clinically speaking, this fact hardly interferes with the endoscopic diagnosis of aGVHD. After onset of diarrhea as the leading symptom, the first diagnostic measure is the microbiological examination of stool, followed by endoscopy 1 or 2 days later. Thus, the microbiological results are already available when endoscopy is performed.
In this paper, we show data on the distribution of histological lesions of aGVHD along the GIT and its diagnostic implications, observing clinically important results: (1) aGVHD grade 4 is the most frequent type of involvement in aGVHD in the small bowel (meaning the duodenum or terminal ileum), (2) in about 20% of cases of gastrointestinal aGVHD grade ⩾2 in the lower GIT, lesions were detected during ileo-colonoscopy only in the terminal ileum and not in the colon. Therefore, if an aGVHD is suspected because of clinical symptoms, the terminal ileum must be inspected if the colon is free of typical lesions, and (3) if the terminal ileum can be examined during colonoscopy, gastro-duodenoscopy yields no additional information. These results lead us to conclude that the duodenum and terminal ileum behave in parallel when affected by aGVHD. A combination of recto-sigmoidoscopy and gastro-duodenoscopy has nearly the same diagnostic yield (92%) as colonoscopy in conjunction with inspection of the terminal ileum (100%). Nevertheless, it is essential to confirm the data by a prospective study in a larger cohort.
We are aware the fact that our study has all the limitations of a retrospective analysis. Nevertheless, the good correlation between the macroscopic and the histological grading has a solid base. Histological criteria are generally accepted, our macroscopic criteria have been defined before the evaluation (there was only a slight modification of the criteria established by ourselves in 1994), and macroscopic and histological feature could be compared in the same region of the affected gut. It may be a shortcoming that the number of biopsies taken during the endoscopy was relatively small. This may have led to underestimation of the histological degree of aGVHD. However, there is a good correlation between macroscopic and histological grading. We do not intend to discourage hematologists or gastroenterologists to take biopsies. On the contrary, we are supporting the conclusions already drawn by Ponec et al.37 According to them endoscopy and histology are complementary. And considering that histology itself has got some limitations, endoscopy can give an important contribution for diagnosis finding.
It may be argued due to the relatively high prevalence of higher-grade endoscopic lesions in our patients that we evaluated patients with a more progressed aGVHD than other authors did. One could divide the group of patients into those with early or late or very late aGVHD. But this is beyond the scope of the manuscript, which describes and evaluates macroscopic findings and correlates them with histology. Differentiation of patients in different groups would reduce the validity of the statistical evaluation without gain of additional information.
Conclusion
The macroscopic aspect of the GIT, in particular the lower GIT, has high validity for diagnosing intestinal aGVHD. Using the ‘Freiburg Criteria’ in ileo-colonoscopy macroscopy facilitates the differentiation between no aGVHD or mild grade 1 aGVHD, and the higher grades ⩾2, which require intensification of immunosuppression. We suggest the following approach in suspected intestinal aGVHD: if intestinal aGVHD is suspected due to diarrhea a stool specimen should be tested for intestinal pathogens (including Clostridium difficile, CMV and cryptosporidia) as soon as possible. The patient should be prepared for colonoscopy, which will be performed the next day. Ideally, the microbial results are available when colonoscopy is performed. If the macroscopic aspect of the colon is typical for aGVHD ⩾2, an ileoscopy need not been done. If the colon appears macroscopically normal the terminal ileum must be inspected. An endoscopic examination of the upper GIT may then be omitted. Nevertheless, biopsies should be taken if ever possible to confirm the macroscopic diagnosis and immunostaining for CMV should be considered.
References
Jenq RR, van den Brink MR . Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat Rev Cancer 2010; 10: 213–221.
Prasad VK, Kurtzberg J . Transplant outcomes in mucopolysaccharidoses. Semin Hematol 2010; 47: 59–69.
Hough R, Rocha V . Transplant outcomes in acute leukemia II. Semin Hematol 2010; 47: 51–58.
MacMillan ML, Walters MC, Gluckman E . Transplant outcomes in bone marrow failure syndromes and hemoglobinopathies. Semin Hematol 2010; 47: 37–45.
Kohn DB . Update on gene therapy for immunodeficiencies. Clin Immunol 2010; 135: 247–254.
Gratwohl A, Heim D . Current role of stem cell transplantation in chronic myeloid leukaemia. Best Pract Res Clin Haematol 2009; 22: 431–443.
Appelbaum FR . Haematopoietic cell transplantation as immunotherapy. Nature 2001; 411: 385–389.
Wagner Jr JE, Vogelsang GB, Beschorner WE . Pathogenesis and pathology of graft-vs-host disease. Am J Pediatr Hematol Oncol 1989; 11: 196–212.
Ferrara JL, Levine JE, Reddy P, Holler E . Graft-versus-host disease. Lancet 2009; 373: 1550–1561.
Shlomchik WD . Graft-versus-host disease. Nat Rev Immunol 2007; 7: 340–352.
Paczesny S, Hanauer D, Sun Y, Reddy P . New perspectives on the biology of acute GVHD. Bone Marrow Transplant 2010; 45: 1–11.
Washington K, Jagasia M . Pathology of graft-versus-host disease in the gastrointestinal tract. Hum Pathol 2009; 40: 909–917.
Couriel D, Caldera H, Champlin R, Komanduri K . Acute graft-versus-host disease: pathophysiology, clinical manifestations, and management. Cancer 2004; 101: 1936–1946.
Ross WA, Ghosh S, Dekovich AA, Liu S, Ayers GD, Cleary KR et al. Endoscopic biopsy diagnosis of acute gastrointestinal graft-versus-host disease: rectosigmoid biopsies are more sensitive than upper gastrointestinal biopsies. Am J Gastroenterol 2008; 103: 982–989.
McDonald GB, Shulman HM, Sullivan KM, Spencer GD . Intestinal and hepatic complications of human bone marrow transplantation. Part I. Gastroenterology 1986; 90: 460–477.
McDonald GB, Shulman HM, Sullivan KM, Spencer GD . Intestinal and hepatic complications of human bone marrow transplantation. Part II. Gastroenterology 1986; 90: 770–784.
Deeg HJ, Antin JH . The clinical spectrum of acute graft-versus-host disease. Semin Hematol 2006; 43: 24–31.
McDonald GB . Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology 2010; 51: 1450–1460.
Lee SJ, Vogelsang G, Gilman A, Weisdorf DJ, Pavletic S, Antin JH et al. A survey of diagnosis, management, and grading of chronic GVHD. Biol Blood Marrow Transplant 2002; 8: 32–39.
Patey-Mariaud de SN, Reijasse D, Verkarre V, Canioni D, Colomb V, Haddad E et al. Chronic intestinal graft-versus-host disease: clinical, histological and immunohistochemical analysis of 17 children. Bone Marrow Transplant 2002; 29: 223–230.
Akpek G, Chinratanalab W, Lee LA, Torbenson M, Hallick JP, Anders V et al. Gastrointestinal involvement in chronic graft-versus-host disease: a clinicopathologic study. Biol Blood Marrow Transplant 2003; 9: 46–51.
McDonald GB, Sullivan KM, Schuffler MD, Shulman HM, Thomas ED . Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology 1981; 80: 914–921.
Sale GE, Shulman HM, McDonald GB, Thomas ED . Gastrointestinal graft-versus-host disease in man. A clinicopathologic study of the rectal biopsy. Am J Surg Pathol 1979; 3: 291–299.
Epstein RJ, McDonald GB, Sale GE, Shulman HM, Thomas ED . The diagnostic accuracy of the rectal biopsy in acute graft-versus-host disease: a prospective study of thirteen patients. Gastroenterology 1980; 78: 764–771.
Chao NJ, Chen BJ . Prophylaxis and treatment of acute graft-versus-host disease. Semin Hematol 2006; 43: 32–41.
Shen B, Khan K, Ikenberry SO, Anderson MA, Banerjee S, Baron T et al. The role of endoscopy in the management of patients with diarrhea. Gastrointest Endosc 2010; 71: 887–892.
Cox GJ, Matsui SM, Lo RS, Hinds M, Bowden RA, Hackman RC et al. Etiology and outcome of diarrhea after marrow transplantation: a prospective study. Gastroenterology 1994; 107: 1398–1407.
Kreisel W, Herbst EW, Schwind B, Ochs A, Olschewski M, Köchling G et al. Diagnosis and grading of acute graft-versus-host disease following allogeneic bone marrow transplantation by sigmoidoscopy. Eur J Gastroenterol Hepatol 1994; 6: 723–729.
Xu CF, Zhu LX, Xu XM, Chen WC, Wu DP . Endoscopic diagnosis of gastrointestinal graft-versus-host disease. World J Gastroenterol 2008; 14: 2262–2267.
Cheung DY, Kim JI, Kim SS, Sung HY, Cho SH, Park SH et al. Endoscopic evaluation in gastrointestinal graft-versus-host disease: comparisons with histological findings. Dig Dis Sci 2008; 53: 2947–2954.
Thompson B, Salzman D, Steinhauer J, Lazenby AJ, Wilcox CM . Prospective endoscopic evaluation for gastrointestinal graft-versus-host disease: determination of the best diagnostic approach. Bone Marrow Transplant 2006; 38: 371–376.
Cruz-Correa M, Poonawala A, Abraham SC, Wu TT, Zahurak M, Vogelsang G et al. Endoscopic findings predict the histologic diagnosis in gastrointestinal graft-versus-host disease. Endoscopy 2002; 34: 808–813.
Roy J, Snover D, Weisdorf S, Mulvahill A, Filipovich A, Weisdorf D . Simultaneous upper and lower endoscopic biopsy in the diagnosis of intestinal graft-versus-host disease. Transplantation 1991; 51: 642–646.
Weisdorf DJ, Snover DC, Haake R, Miller WJ, McGlave PB, Blazar B et al. Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy. Blood 1990; 76: 624–629.
Snover DC . Mucosal damage simulating acute graft-versus-host reaction in cytomegalovirus colitis. Transplantation 1985; 39: 669–670.
Müller CI, Zeiser R, Grüllich C, Finke J, Bertz H, Schmitt-Gräff A et al. Intestinal cryptosporidiosis mimicking acute graft-versus-host disease following matched unrelated hematopoietic stem cell transplantation. Transplantation 2004; 77: 1478–1479.
Ponec RJ, Hackman RC, McDonald GB . Endoscopic and histologic diagnosis of intestinal graft-versus-host disease after marrow transplantation. Gastrointest Endosc 1999; 49: 612–621.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Kreisel, W., Dahlberg, M., Bertz, H. et al. Endoscopic diagnosis of acute intestinal GVHD following allogeneic hematopoietic SCT: a retrospective analysis in 175 patients. Bone Marrow Transplant 47, 430–438 (2012). https://doi.org/10.1038/bmt.2011.137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2011.137
Keywords
This article is cited by
-
Endoscopic manifestation of intestinal transplant-associated microangiopathy after stem cell transplantation
BMC Gastroenterology (2024)
-
PET assessment of acute gastrointestinal graft versus host disease
Bone Marrow Transplantation (2023)
-
Terminal ileum is the most sensitive site for the histologic diagnosis of grade 4 graft-versus-host disease (GvHD) in the lower GI tract and is a harbinger of poor outcome
Virchows Archiv (2021)
-
Histological and magnified endoscopic evaluation of villous atrophy in gastrointestinal graft-versus-host disease
Annals of Hematology (2020)
-
Ileostomy for steroid-resistant acute graft-versus-host disease of the gastrointestinal tract
Annals of Hematology (2019)