Abstract
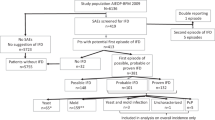
Invasive fungal disease (IFD) causes significant morbidity and mortality among children undergoing allo-SCT. In this prospective pilot study, we analyze voriconazole as primary antifungal prophylaxis. From October 2004 to July 2010, 56 children <18 years of age were enrolled in this study. Patients received voriconazole doses of 5 mg/kg per 12 h (n=23) or 7 mg/kg per 12 h (n=33), with a limiting dose of 200 mg/12 h, from day −1 to day +75 or later in patients with active acute GVHD. Patients were followed up for IFD for 6 months. In this series, 37 (66.1%) patients successfully completed treatment (85.7% during neutropenic period) without empirical or preemptive antifungal therapy, adverse effects or IFD. Nine (16.1%) children needed preemptive (n=2) or empirical (n=7) antifungal therapy, and one (1.8%) of them developed a fatal probable IFD during the study period. A total of 10 (17.8%) children developed adverse effects related to voriconazole prophylaxis, leading to definitive withdrawal on median day 26.5 (in 7 patients after granulocytic recovery). The most frequent adverse effect was persistent elevation of hepatic enzymes in seven (12.5%) children. There were no differences between doses of 5 and 7 mg/kg per 12 h. Our results suggest that voriconazole can be safely used as primary antifungal prophylaxis in children undergoing allo-SCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 2010; 50: 1091–1100.
Rubio PM, Sevilla J, Gonzalez-Vicent M, Lassaletta A, Cuenca-Estrella M, Díaz MA et al. Increasing incidence of invasive aspergillosis in pediatric hematology oncology patients over the last decade: a retrospective single centre study. J Pediatr Hematol Oncol 2009; 31: 642–646.
Lin SJ, Schranz J, Teutsch SM . Aspergillosis case-fatality rate systematic review of literature. Clin Infect Dis 2001; 32: 358–366.
Leather JL, Wingard JR . New strategies of antifungal therapy in hematopoietic stem cell transplant recipients and patients with hematological malignancies. Blood Rev 2006; 20: 267–287.
Dvorak CC, Steinbach WJ, Brown JM, Agarwal R . Risks and outcomes of invasive fungal infections in pediatric patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2005; 36: 621–629.
Baddley JW, Andes DR, Marr KA, Kontoyiannis DP, Alexander BD, Kauffman CA et al. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infec Dis 2010; 50: 1559–1567.
Thursky K, Byrnes G, Grigg A, Szer J, Slavin M . Risk factors for post-engraftment invasive aspergillosis in allogeneic stem cell transplantation. Bone Marrow Transplant 2004; 34: 115–121.
Steinbach WJ, Addison RM, McLAughlin L, Gerrald Q, Martin PL, Driscoll T et al. Prospective aspergillus galactomannan antigen testing in pediatric hematopoietic stem cell transplant recipients. Pediatr Infect Dis J 2007; 26: 558–564.
Hayden R, Pounds S, Knapp K, Petraitiene R, Schaufele RL, Sein T et al. Galactomannan antigenemia in pediatric oncology patients with invasive aspergillosis. Pediatr Infect Dis J 2008; 27: 815–819.
Smith PB, Benjamin DK, Alexander BD, Johnson MD, Finkelman MA, Steinbach WJ . Quantification of 1,3-β-d-glucan levels in children: preliminary data for diagnostic use of the β-glucan assay in a pediatric setting. Clin Vaccine Immunol 2007; 14: 924–925.
Armenian AH, Nash KA, Kapoor N, Franklin JL, Gaynon PS, Ross LA et al. Prospective monitoring for invasive aspergillosis using galactomannan and polymerase chain reaction in high risk pediatric patients. J Pediatr Hematol Oncol 2009; 31: 920–926.
Maertens JA, Madero L, Reilly AF, Lehrnbecher T, Groll AH, Jafri HS et al. A randomized, double-blind, multicenter study of caspofungin versus liposomal amphotericin B for empiric antifungal therapy in pediatric patients with persistent fever and neutropenia. Pediatr Infect Dis J 2010; 29: 415–420.
Maertens J, Theunissen K, Verhoef G, Verschakelen J, Lagrou K, Verbeken E et al. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis 2005; 41: 1242–1250.
Kanda Y, Yamamoto R, Chizuka A, Hamaki T, Suguro M, Arai C et al. Prophylactic action of oral fluconazole against fungal infection in neutropenic patients. A meta-analysis of 16 randomized, controlled trials. Cancer 2000; 89: 1611–1625.
Baddley JW, Marr KA, Andes DR, Walsh TJ, Kauffman CA, Kontoyiannis DP et al. Patterns of susceptibility of aspergillus isolates recovered from patients enrolled in the Transplant-Associated Infection Surveillance Network. J Clin Microbiol 2009; 47: 3271–3275.
Marr KA, Crippa F, Leisenring W, Hoyle M, Boeckh M, Balajee SA et al. Itraconazole versus fluconazole for prevention of fangal infections in patients receiving allogeneic stem cell transplants. Blood 2004; 103: 1527–1533.
Torres A, Serrano J, Rojas R, Martin V, Martin C, Tabares S et al. Voriconazole as primary antifungal prophylaxis in patients with neutropenia after hematopoietic stem cell transplantation or chemotherapy for acute myeloid leukemia. Eur J Haematol 2010; 84: 371–373.
Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR et al. Posaconazole or fuconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 2007; 356: 335–347.
van Burik JA, Ratanatharathorn V, Stepan DE, Miller CB, Lipton JH, Vesole DH et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 2004; 39: 1407–1416.
Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Baden LR et al. Randomized double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoetic cell transplantation. Blood 2010; 115: 5111–5118.
Mehta PA, Vinks AA, Filipovich A, Bleesing J, Jodele S, Jordan MB et al. Alternate-day micafungin antifungal prophylaxis in pediatric patients undergoing hematopoietic stem cell transplantation: a pharmacokinetic study. Biol Blood Marrow Transplant 2010; 16: 1458–1462.
Roman E, Osunkwo I, Militano O, Cooney E, van de Ven C, Cairo MS . Liposomal amphotericin B prophylaxis of invasive mold infections in children post allogeneic stem cell transplantation. Pediatr Blood Cancer 2008; 50: 325–330.
Hugues WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T et al. Guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 2002; 34: 730–751.
Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002; 34: 7–14.
de Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycosis Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46: 1813–1821.
Sulahian A, Tabouret M, Ribaud P, Sarfati J, Gluckman E, Latge JP et al. Comparison of an enzyme immunoassay and a latex agglutination test for detection of galactomannan in the diagnosis of invasive aspergillosis. Eur J Clin Microbiol Infect Dis 1996; 15: 139–145.
Mennink-Kersten MA, Donnelly JP, Verweij PE . Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect Dis 2004; 4: 349–357.
Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 1992; 326: 845–851.
Kolve H, Ahlke E, Fegeler W, Ritter J, Jurgens H, Groll AH . Safety, tolerance and outcome of treatment with liposomal amphotericin B in paediatric patients with cancer or undergoing haematopoietic stem cell transplantation. J Antimicrob Chemother 2009; 64: 383–387.
Trifilio SM, Bennett CL, Yarnold PR, McKoy JM, Parada J, Mehta J et al. Breakthrough zygomycosis after voriconazole administration among patients with hematological malignancies who receive hematopoietic stem-cell transplants or intensive chemotherapy. Bone Marrow Transplant 2007; 39: 425–429.
Amigues I, Cohen N, Chung D, Seo SK, Plescia C, Jakubowski A et al. Hepatic safety of voriconazole after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2010; 16: 46–52.
Karlsson MO, Lutsar I, Milligan PA . Population pharmacokinetic analysis of voriconazole plasma concentration data from pediatric studies. Antimicrob Agents Chemother 2009; 53: 935–944.
Groll AH, Kolve H, Ehlert K, Paulussen M, Vormoor J . Pharmacokinetic interaction between voriconazole and ciclosporin A following allogeneic bone marrow transplantation. J Antimicrob Chemother 2004; 53: 113–114.
Kontoyiannis DP . Antifungal prophylaxis in hematopoietic stem cell transplant recipients: the unfinished tale of imperfect success. Bone Marrow Transplant 2011; 46: 165–173.
Acknowledgements
This study was supported by Asociacion Medicina e Investigación (AMI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JR Molina has received honoraria for speaking at medical education events supported by Gilead Science and Pfizer. R Rojas has received honoraria for speaking at symposia organized by Gilead Science and Merck Sharp and Dohme (MSD). A Torres has received honoraria for participation as a speaker at a medical education event and symposia supported by Gilead, Pfizer and MSD. All the other authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Molina, J., Serrano, J., Sánchez-García, J. et al. Voriconazole as primary antifungal prophylaxis in children undergoing allo-SCT. Bone Marrow Transplant 47, 562–567 (2012). https://doi.org/10.1038/bmt.2011.111
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2011.111
Keywords
This article is cited by
-
Primary and Secondary Antifungal Prophylaxis in the Immunocompromised Child: Where do we Stand?
Current Fungal Infection Reports (2013)
-
Pharmacokinetics, Safety and Efficacy of Voriconazole in Pediatric Patients: An Update
Current Fungal Infection Reports (2012)