Abstract
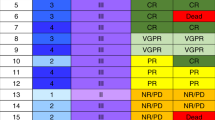
The efficacy and safety of CD52 antibody alemtuzumab to treat severe acute GVHD in 18 consecutive patients refractory to standard high-dose corticosteroid therapy is reported. Patients (age range 13–68 years) had developed acute GVHD grade III and IV with gut and/or liver involvement after stem cell transplantation from family donors (n=7) or HLA-matched unrelated donors (n=11), including five donors with one or two HLA mismatches. Initially, in three patients, start doses of alemtuzumab in the range of 70–80 mg were applied and repeated after 3 to 4 weeks. Impressive responses were seen, but virus reactivation and bacterial infections were frequent. In an attempt to reduce this complication, the next nine patients received a reduced starting dose of 20–33 mg, and the last six patients received 3–13 mg repeated every 2–3 weeks. Seventeen of 18 patients responded to alemtuzumab, six patients are alive with a median follow-up of 108 weeks. Chronic GVHD was observed frequently. Although pronounced lymphocyte depletion requiring close monitoring for signs of infections seems inevitable for efficacy, alemtuzumab given in moderate doses has a substantial activity not only in intestinal but also in severe acute GVHD of the liver.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ho VT, Cutler C . Current and novel therapies in acute GVHD. Best Pract Res Clin Haematol 2008; 21: 223–237.
Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood 2002; 99: 3554–3561.
Dearden C . Alemtuzumab in peripheral T-cell malignancies. Cancer Biother Radiopharm 2004; 19: 391–398.
Enblad G, Hagberg H, Erlanson M, Lundin J, MacDonald AP, Repp R et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood 2004; 103: 2920–2924.
Lundin J, Hagberg H, Repp R, Cavallin-Stahl E, Freden S, Juliusson G et al. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood 2003; 101: 4267–4272.
Reiff A . A review of Campath in autoimmune disease: biologic therapy in the gray zone between immunosuppression and immunoablation. Hematology 2005; 10: 79–93.
Heit W, Bunjes D, Wiesneth M, Schmeiser T, Arnold R, Hale G et al. Ex vivo T-cell depletion with the monoclonal antibody Campath-1 plus human complement effectively prevents acute graft-versus-host disease in allogeneic bone marrow transplantation. Br J Haematol 1986; 64: 479–486.
Kottaridis PD, Milligan DW, Chopra R, Chakraverty RK, Chakrabarti S, Robinson S et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood 2000; 96: 2419–2425.
Wandroo F, Auguston B, Cook M, Craddock C, Mahendra P . Successful use of Campath-1H in the treatment of steroid refractory liver GvHD. Bone Marrow Transplant 2004; 34: 285–287.
Carella AM, Beltrami G, Scalzulli PR, Carella Jr AM, Corsetti MT . Alemtuzumab can successfully treat steroid-refractory acute graft-versus-host disease (aGVHD). Bone Marrow Transplant 2004; 33: 131–132.
Gomez-Almaguer D, Ruiz-Arguelles GJ, del Carmen Tarin-Arzaga L, Gonzalez-Llano O, Gutierrez-Aguirre H, Cantu-Rodriguez O et al. Alemtuzumab for the treatment of steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant 2008; 14: 10–15.
Ruiz-Arguelles GJ, Gil-Beristain J, Magana M, Ruiz-Delgado GJ . Alemtuzumab-induced resolution of refractory cutaneous chronic graft-versus-host disease. Biol Blood Marrow Transplant 2008; 14: 7–9.
Martinez C, Solano C, Ferra C, Sampol A, Valcarcel D, Perez-Simon JA . Alemtuzumab as treatment of steroid-refractory acute graft-versus-host disease: results of a phase II study. Biol Blood Marrow Transplant 2009; 15: 639–642.
Schnitzler M, Hasskarl J, Egger M, Bertz H, Finke J . Successful treatment of severe acute intestinal graft-versus-host resistant to systemic and topical steroids with alemtuzumab. Biol Blood Marrow Transplant 2009; 15: 910–918.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Schub, N., Günther, A., Schrauder, A. et al. Therapy of steroid-refractory acute GVHD with CD52 antibody alemtuzumab is effective. Bone Marrow Transplant 46, 143–147 (2011). https://doi.org/10.1038/bmt.2010.68
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2010.68
Keywords
This article is cited by
-
Alemtuzumab treatment for steroid-refractory acute graft-versus-host disease leads to severe immunosuppression but not to relapse of malignant disease
Bone Marrow Transplantation (2024)
-
Ruxolitinib is an effective salvage treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation without posttransplant cyclophosphamide
Annals of Hematology (2021)
-
Ruxolitinib treatment for SR-aGVHD in patients with EBV-HLH undergoing allo-HSCT
Annals of Hematology (2020)
-
Inpatient Management of Mucocutaneous GVHD
Current Dermatology Reports (2019)
-
Pentostatin therapy for steroid-refractory acute graft versus host disease: identifying those who may benefit
Bone Marrow Transplantation (2018)