Abstract
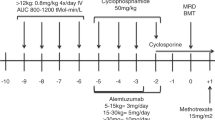
Busulfan influences engraftment and toxicities during hematopoietic stem cell transplantation (HSCT). We report our single-institution experience of targeted busulfan therapy for myeloablative, matched sibling donor (MSD) HSCT for sickle cell disease (SCD) and assess the relationships of busulfan levels to engraftment and toxicities. Twenty-seven patients with SCD underwent MSD HSCT from 1993 to 2007, 25 with busulfan measurements. The conditioning regimen was busulfan (initial dose 0.875 mg/kg for 16 doses), CY and antithymocyte globulin. Busulfan area under curve (AUC) was determined with the first dose, and dose adjustments were made to target the desired AUC range. Median AUC was 963 μmol min/L (range 780–1305 μmol min/L). Engraftment occurred in all cases: 21 (84%) full donor chimerism (>95% donor cells), 4 (16%) partial donor chimerism. Hepatic veno-occlusive disease (VOD) occurred in 8 (32%) patients. Lower AUC was seen with partial donor chimerism (862±73 μmol min/L) versus full donor chimerism (AUC 1018±122 μmol min/L) (P=0.022). VOD was not associated with busulfan AUC (P=0.153). Of 25 patients, 24 survived with median follow-up of 4.9 years. Our experience shows that targeting busulfan AUC above the range used in previous multicenter trials appears safe and may contribute to sustained engraftment in SCD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tutschka PJ, Santon GW . Bone marrow transplantation in the busulfin-treated rat. III. Relationship between myelosuppression and immunosuppression for conditioning bone marrow recipients. Transplantation 1977; 24: 52–62.
Slattery JT, Sanders JE, Buckner CD, Schaffer RL, Lambert KW, Langer FP et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant 1995; 16: 31–42.
Chandy M, Balasubramanian P, Ramachandran SV, Mathews V, George B, Dennison D et al. Randomized trial of two different conditioning regimens for bone marrow transplantation in thalassemia—the role of busulfan pharmacokinetics in determining outcome. Bone Marrow Transplant 2005; 36: 839–845.
Vassal G, Deroussent A, Hartmann O, Challine D, Benhamou E, Valteau-Couanet D et al. Dose-dependent neurotoxicity of high-dose busulfan in children: a clinical and pharmacological study. Cancer Res 1990; 50: 6203–6207.
Grochow LB, Jones RJ, Brundrett RB, Braine HG, Chen TL, Saral R et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1989; 25: 55–61.
Ljungman P, Hassan M, Bekassy AN, Ringden O, Oberg G . High busulfan concentrations are associated with increased transplant-related mortality in allogeneic bone marrow transplant patients. Bone Marrow Transplant 1997; 20: 909–913.
Walters MC, Patience M, Leisenring W, Rogers ZR, Aquino VM, Buchanan GR et al. Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant 2001; 7: 665–673.
McCune JS, Gooley T, Gibbs JP, Sanders JE, Petersdorf EW, Appelbaum FR et al. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 2002; 30: 167–173.
Schechter T, Finkelstein Y, Doyle J, Verjee Z, Moretti M, Koren G et al. Pharmacokinetic disposition and clinical outcomes in infants and children receiving intravenous busulfan for allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2007; 13: 307–314.
Zwaveling J, Bredius RG, Cremers SC, Ball LM, Lankester AC, Teepe-Twiss IM et al. Intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics in association with early clinical outcome and toxicity. Bone Marrow Transplant 2005; 35: 17–23.
Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant 2002; 8: 477–485.
Bolinger AM, Zangwill AB, Slattery JT, Risler LJ, Sultan DH, Glidden DV et al. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation. Bone Marrow Transplant 2001; 28: 1013–1018.
Walters MC, Storb R, Patience M, Leisenring W, Taylor T, Sanders JE et al. Impact of bone marrow transplantation for symptomatic sickle cell disease: an interim report. Multicenter investigation of bone marrow transplantation for sickle cell disease. Blood 2000; 95: 1918–1924.
Walters MC, Hardy K, Edwards S, Adamkiewicz T, Barkovich J, Bernaudin F et al. Pulmonary, gonadal and central nervous system status after bone marrow transplantation for sickle cell disease. Biol Blood Marrow Transplant 2010; 16: 263–272.
Walters MC, Patience M, Leisenring W, Eckman JR, Scott JP, Mentzer WC et al. Bone marrow transplantation for sickle cell disease. N Engl J Med 1996; 335: 369–376.
Grochow LB . Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol 1993; 20 (4 Suppl 4): 18–25; quiz 26.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED . Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology 1984; 4: 116–122.
Bernaudin F, Socie G, Kuentz M, Chevret S, Duval M, Bertrand Y et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood 2007; 110: 2749–2756.
Andreani M, Nesci S, Lucarelli G, Tonucci P, Rapa S, Angelucci E et al. Long-term survival of ex-thalassemic patients with persistent mixed chimerism after bone marrow transplantation. Bone Marrow Transplant 2000; 25: 401–404.
Amrolia PJ, Vulliamy T, Vassiliou G, Lawson S, Bryon J, Kaeda J et al. Analysis of chimerism in thalassaemic children undergoing stem cell transplantation. Br J Haematol 2001; 114: 219–225.
Krishnamurti L, Kharbanda S, Biernacki MA, Zhang W, Baker KS, Wagner JE et al. Stable long-term donor engraftment following reduced-intensity hematopoietic cell transplantation for sickle cell disease. Biol Blood Marrow Transplant 2008; 14: 1270–1278.
Lucarelli G, Galimberti M, Polchi P, Angelucci E, Baronciani D, Giardini C et al. Bone marrow transplantation in patients with thalassemia. N Engl J Med 1990; 322: 417–421.
Bhatia M, Walters MC . Hematopoietic cell transplantation for thalassemia and sickle cell disease: past, present and future. Bone Marrow Transplant 2008; 41: 109–117.
Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant 1996; 17: 225–230.
Grochow LB, Krivit W, Whitley CB, Blazar B . Busulfan disposition in children. Blood 1990; 75: 1723–1727.
Yeager AM, Wagner Jr JE, Graham ML, Jones RJ, Santos GW, Grochow LB . Optimization of busulfan dosage in children undergoing bone marrow transplantation: a pharmacokinetic study of dose escalation. Blood 1992; 80: 2425–2428.
McPherson ME, Anderson AR, Haight AE, Jessup P, Castillejo MI, Hillyer CD et al. Transfusion management of sickle cell patients during bone marrow transplantation with matched sibling donor. Transfusion 2009; 49: 1977–1986.
Eggleston B, Patience M, Edwards S, Adamkiewicz T, Buchanan GR, Davies SC et al. Effect of myeloablative bone marrow transplantation on growth in children with sickle cell anaemia: results of the multicenter study of haematopoietic cell transplantation for sickle cell anaemia. Br J Haematol 2007; 136: 673–676.
Rozman C, Carreras E, Qian C, Gale RP, Bortin MM, Rowlings PA et al. Risk factors for hepatic veno-occlusive disease following HLA-identical sibling bone marrow transplants for leukemia. Bone Marrow Transplant 1996; 17: 75–80.
Hassan M, Oberg G, Bjorkholm M, Wallin I, Lindgren M . Influence of prophylactic anticonvulsant therapy on high-dose busulphan kinetics. Cancer Chemother Pharmacol 1993; 33: 181–186.
Kashyap A, Wingard J, Cagnoni P, Roy J, Tarantolo S, Hu W et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant 2002; 8: 493–500.
Bearman SI . The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood 1995; 85: 3005–3020.
Ciurea SO, Andersson BS . Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2009; 15: 523–536.
Morris CR . Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematology Am Soc Hematol Educ Program 2008; 2008: 177–185.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
McPherson, M., Hutcherson, D., Olson, E. et al. Safety and efficacy of targeted busulfan therapy in children undergoing myeloablative matched sibling donor BMT for sickle cell disease. Bone Marrow Transplant 46, 27–33 (2011). https://doi.org/10.1038/bmt.2010.60
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2010.60
Keywords
This article is cited by
-
Targeted Busulfan therapy with a steady-state concentration of 600–700 ng/mL in patients with sickle cell disease receiving HLA-identical sibling bone marrow transplant
Bone Marrow Transplantation (2014)
-
Hematopoietic SCT for the Black African and non-Black African variants of sickle cell anemia
Bone Marrow Transplantation (2014)
-
Allogeneic cellular gene therapy in hemoglobinopathies—evaluation of hematopoietic SCT in sickle cell anemia
Bone Marrow Transplantation (2012)