Abstract
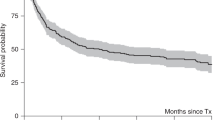
Women with breast cancer who receive adjuvant therapy are at risk for developing therapy-related myelodysplastic syndrome (MDS) or AML (tMDS/AML). Patients with tMDS/AML are often referred for consideration of allogeneic hematopoietic SCT (HSCT). However, the outcomes of HSCT in such patients have not been well described. We report a retrospective study of all women who were treated with HSCT for MDS or AML at our institution between 1991 and 2008. We compared the transplantation outcomes for 24 women with a history of breast cancer with those for 271 women with de novo disease. Three-year OS and disease-free survival (DFS) for patients with a history of breast cancer were 41 and 45%, respectively. The cumulative incidences of tMDS/AML relapse and non-relapse mortality (NRM) were 38 and 17%, respectively. Those outcomes were very similar to those of patients with de novo disease. In multivariable analyses, a history of breast cancer had no impact on OS, DFS, relapse or NRM. A significant proportion of women with tAML/MDS after breast cancer treatment experience DFS after HSCT, similar to that of patients with de novo MDS or AML. This justifies consideration of HSCT for selected patients in this setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Curtis RE, Boice Jr JD, Stovall M, Bernstein L, Greenberg RS, Flannery JT et al. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med 1992; 326: 1745–1751.
Curtis RE, Boice Jr JD, Stovall M, Bernstein L, Holowaty E, Karjalainen S et al. Relationship of leukemia risk to radiation dose following cancer of the uterine corpus. J Natl Cancer Inst 1994; 86: 1315–1324.
Cadman EC, Capizzi RL, Bertino JR . Acute nonlymphocytic leukemia: a delayed complication of Hodgkin's disease therapy: analysis of 109 cases. Cancer 1977; 40: 1280–1296.
Arseneau JC, Sponzo RW, Levin DL, Schnipper LE, Bonner H, Young RC et al. Nonlymphomatous malignant tumors complicating Hodgkin's disease. Possible association with intensive therapy. N Engl J Med 1972; 287: 1119–1122.
Karchmer RK, Amare M, Larsen WE, Mallouk AG, Caldwell GG . Alkylating agents as leukemogens in multiple myeloma. Cancer 1974; 33: 1103–1107.
Ratain MJ, Kaminer LS, Bitran JD, Larson RA, Le Beau MM, Skosey C et al. Acute nonlymphocytic leukemia following etoposide and cisplatin combination chemotherapy for advanced non-small-cell carcinoma of the lung. Blood 1987; 70: 1412–1417.
Rowley JD, Golomb HM, Vardiman JW . Nonrandom chromosome abnormalities in acute leukemia and dysmyelopoietic syndromes in patients with previously treated malignant disease. Blood 1981; 58: 759–767.
Fisher B, Rockette H, Fisher ER, Wickerham DL, Redmond C, Brown A . Leukemia in breast cancer patients following adjuvant chemotherapy or postoperative radiation: the NSABP experience. J Clin Oncol 1985; 3: 1640–1658.
Smith RE, Bryant J, DeCillis A, Anderson S . Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol 2003; 21: 1195–1204.
Schaapveld M, Visser O, Louwman MJ, de Vries EG, Willemse PH, Otter R et al. Risk of new primary nonbreast cancers after breast cancer treatment: a Dutch population-based study. J Clin Oncol 2008; 26: 1239–1246.
Cluze C, Delafosse P, Seigneurin A, Colonna M . Incidence of second cancer within 5 years of diagnosis of a breast, prostate or colorectal cancer: a population-based study. Eur J Cancer Prev 2009; 18: 343–348.
Pedersen-Bjergaard J, Philip P, Larsen SO, Jensen G, Byrsting K . Chromosome aberrations and prognostic factors in therapy-related myelodysplasia and acute nonlymphocytic leukemia. Blood 1990; 76: 1083–1091.
Goldstone A, Burnett A, Avivi I, Hills R, Wheatley K . Secondary acute myeloid leukemia has a worse outcome than de novo AML, even taking into account cytogenetics and age: AML 10, 11, 12 MRC Trials. Blood 2002; 100: 88a.
Josting A, Wiedenmann S, Franklin J, May M, Sieber M, Wolf J et al. Secondary myeloid leukemia and myelodysplastic syndromes in patients treated for Hodgkin's disease: a report from the German Hodgkin's Lymphoma Study Group. J Clin Oncol 2003; 21: 3440–3446.
Kern W, Haferlach T, Schnittger S, Hiddemann W, Schoch C . Prognosis in therapy-related acute myeloid leukemia and impact of karyotype. J Clin Oncol 2004; 22: 2510–2511.
Mauritzson N, Albin M, Rylander L, Billstrom R, Ahlgren T, Mikoczy Z et al. Pooled analysis of clinical and cytogenetic features in treatment-related and de novo adult acute myeloid leukemia and myelodysplastic syndromes based on a consecutive series of 761 patients analyzed 1976–1993 and on 5098 unselected cases reported in the literature 1974–2001. Leukemia 2002; 16: 2366–2378.
Smith SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood 2003; 102: 43–52.
Kantarjian HM, Keating MJ, Walters RS, Smith TL, Cork A, McCredie KB et al. Therapy-related leukemia and myelodysplastic syndrome: clinical, cytogenetic, and prognostic features. J Clin Oncol 1986; 4: 1748–1757.
Michels SD, McKenna RW, Arthur DC, Brunning RD . Therapy-related acute myeloid leukemia and myelodysplastic syndrome: a clinical and morphologic study of 65 cases. Blood 1985; 65: 1364–1372.
Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood 1998; 92: 2322–2333.
Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood 2002; 100: 4325–4336.
Armand P, Kim HT, DeAngelo DJ, Ho VT, Cutler CS, Stone RM et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant 2007; 13: 655–664.
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89: 2079–2088.
Armand P, Deeg HJ, Kim HT, Lee H, Armistead P, de Lima M et al. Multicenter validation study of a transplantation-specific cytogenetics grouping scheme for patients with myelodysplastic syndromes. Bone Marrow Transplant (e-pub ahead of print 28 September 2009).
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Gray R . A class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of Statistics 1988; 16: 1140–1154.
Fine J, Gray R . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
Hoyle CF, de Bastos M, Wheatley K, Sherrington PD, Fischer PJ, Rees JK et al. AML associated with previous cytotoxic therapy, MDS or myeloproliferative disorders: results from the MRC's 9th AML trial. Br J Haematol 1989; 72: 45–53.
Liu MC, Demetri GD, Berry DA, Norton L, Broadwater G, Robert NJ et al. Dose-escalation of filgrastim does not improve efficacy: clinical tolerability and long-term follow-up on CALGB study 9141 adjuvant chemotherapy for node-positive breast cancer patients using dose-intensified doxorubicin plus cyclophosphamide followed by paclitaxel. Cancer Treat Rev 2008; 34: 223–230.
Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003; 21: 1431–1439.
Hershman D, Neugut AI, Jacobson JS, Wang J, Tsai WY, McBride R et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst 2007; 99: 196–205.
Jones S, Holmes FA, O'Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol 2009; 27: 1177–1183.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Pawlicki M et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (ACT) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (ACTH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 study. Breast Cancer Res Treatment (Abstract) 2005; 94: S5.
Acknowledgements
This work was funded in part by P01 AI 29350 from the National Institute of Allergy and Infectious Diseases. PA is the recipient of a career development award from the Leukemia and Lymphoma Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Armand, P., Kim, H., Mayer, E. et al. Outcome of allo-SCT for women with MDS or AML occurring after breast cancer therapy. Bone Marrow Transplant 45, 1611–1617 (2010). https://doi.org/10.1038/bmt.2010.20
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2010.20
Keywords
This article is cited by
-
Comprehensive analysis of factors impacting risks and outcomes of therapy-related myeloid neoplasms following breast cancer treatment
Leukemia (2016)
-
Long-term follow-up of therapy-related myelodysplasia and AML patients treated with allogeneic hematopoietic cell transplantation
Bone Marrow Transplantation (2016)
-
Carcinomatosis lymphangitis and pleurisy after allo-SCT in two patients with secondary leukemia after breast cancer
Bone Marrow Transplantation (2012)