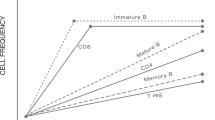
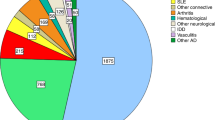
Abstract
Multiple sclerosis (MS) is the leading autoimmune indication for autologous hematopoietic SCT (aHSCT). Patient selection criteria and transplant interventions have been refined through a series of cohort and registry studies. High- and low-intensity chemotherapy-based conditioning regimens have been used, creating trade-offs between toxicity and effectiveness. TBI has been associated with greater toxicity and poor outcomes. aHSCT stops MS relapses and lessens the disability in malignant MS, which otherwise rapidly incapacitates patients. Better responses occur in progressive MS earlier in the disease when it has a more inflammatory nature. aHSCT prevents further disability in many patients, but some actually recover from their infirmities. Current regimens and supportive care result in very low morbidity and mortality. MS patients experience unique complications in addition to the expected toxicities. Cytokines used alone for stem-cell mobilization may induce MS flares but are safe to be used in combination with steroids or cytotoxic agents. Urinary tract infections, herpes virus reactivation and an engraftment syndrome may occur early after aHSCT. Rarely secondary autoimmune diseases have been reported late after HSCT. Increasing experience in caring for patients with MS has reduced the frequency and severity of toxicity. Conceived as an opportunity to ‘reboot’ a tolerant immune system, aHSCT is successful in treating patients with MS that is refractory to conventional immunomodulatory drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Giovannoni G, Ebers G . Multiple sclerosis: the environment and causation. Curr Opin Neurol 2007; 20: 261–268.
Kurtzke JF . Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452.
Degenhardt A, Ramagopalan SV, Scalfari A, Ebers GC . Clinical prognostic factors in multiple sclerosis: a natural history review. Nat Rev Neurol 2009; 5: 672–682.
Filippi M, Horsfield MA, Morrissey SP, MacManus DG, Rudge P, McDonald WI et al. Quantitative brain MRI lesion load predicts the course of clinically isolated syndromes suggestive of multiple sclerosis. Neurology 1994; 44: 635–641.
van Bekkum DW . Stem cell transplantation for autoimmune disorders. Preclinical experiments. Best Pract Res Clin Haematol 2004; 17: 201–222.
Burt RK, Padilla J, Begolka WS, Canto MC, Miller SD . Effect of disease stage on clinical outcome after syngeneic bone marrow transplantation for relapsing experimental autoimmune encephalomyelitis. Blood 1998; 91: 2609–2616.
Cassiani-Ingoni R, Muraro PA, Magnus T, Reichert-Scrivner S, Schmidt J, Huh J et al. Disease progression after bone marrow transplantation in a model of multiple sclerosis is associated with chronic microglial and glial progenitor response. J Neuropathol Exp Neurol 2007; 66: 637–649.
Van Laar JM, Tyndall A . Intense immunosuppression and stem-cell transplantation for patients with severe rheumatic autoimmune disease: a review. Cancer Control 2003; 10: 57–65.
Passweg J, Gratwohl A, Tyndall A . Hematopoietic stem cell transplantation for autoimmune disorders. Curr Opin Hematol 1999; 6: 400–405.
McAllister LD, Beatty PG, Rose J . Allogeneic bone marrow transplant for chronic myelogenous leukemia in a patient with multiple sclerosis. Bone Marrow Transplant 1997; 19: 395–397.
McKendry RJ, Huebsch L, Leclair B . Progression of rheumatoid arthritis following bone marrow transplantation. A case report with a 13-year followup. Arthritis Rheum 1996; 39: 1246–1253.
Lopez-Cubero SO, Sullivan KM, McDonald GB . Course of Crohn's disease after allogeneic marrow transplantation. Gastroenterology 1998; 114: 433–440.
Mancardi GL, Murialdo A, Rossi P, Gualandi F, Martino G, Marmont A et al. Autologous stem cell transplantation as rescue therapy in malignant forms of multiple sclerosis. Mult Scler 2005; 11: 367–371.
Fagius J, Lundgren J, Oberg G . Early highly aggressive MS successfully treated by hematopoietic stem cell transplantation. Mult Scler 2009; 15: 229–237.
Kimiskidis V, Sakellari I, Tsimourtou V, Kapina V, Papagiannopoulos S, Kazis D et al. Autologous stem-cell transplantation in malignant multiple sclerosis: a case with a favorable long-term outcome. Mult Scler 2008; 14: 278–283.
Burt RK, Loh Y, Cohen B, Stefoski D, Balabanov R, Katsamakis G et al. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol 2009; 8: 244–253.
Carreras E, Saiz A, Marín P, Martínez C, Rovira M, Villamor N et al. CD34+ selected autologous peripheral blood stem cell transplantation for multiple sclerosis: report of toxicity and treatment results at one year of follow-up in 15 patients. Haematologica 2003; 88: 306–314.
Saiz A, Blanco Y, Berenguer J, Gómez-Choco M, Carreras E, Arbizu T et al. Clinical outcome 6 years after autologous hematopoietic stem cell transplantation in multiple sclerosis. Neurologia 2008; 23: 405–407.
Fassas A, Anagnostopoulos A, Kazis A, Kapinas K, Sakellari I, Kimiskidis V et al. Autologous stem cell transplantation in progressive multiple sclerosis—an interim analysis of efficacy. J Clin Immunol 2000; 20: 24–30.
Grigg A, Tubridy NJ, Szer J, Mitchell P, Butzkueven H, Shuttleworth P et al. Cladribine followed by autologous stem-cell transplantation in progressive multiple sclerosis. Intern Med J 2004; 34: 66–69.
Hamerschlak N, Rodrigues M, Moraes DA, Oliveira MC, Stracieri AB, Pieroni F et al. Brazilian experience with two conditioning regimens in patients with multiple sclerosis: BEAM/horse ATG and CY/rabbit ATG. Bone Marrow Transplant 2010; 45: 239–248.
Kozák T, Havrdová E, Pit′ha J, Gregora E, Pytlík R, Maaloufová J et al. High-dose immunosuppressive therapy with PBPC support in the treatment of poor risk multiple sclerosis. Bone Marrow Transplant 2000; 25: 525–531.
Ni XS, Ouyang J, Zhu WH, Wang C, Chen B . Autologous hematopoietic stem cell transplantation for progressive multiple sclerosis: report of efficacy and safety at three yr of follow up in 21 patients. Clin Transplant 2006; 20: 485–489.
Openshaw H, Lund BT, Kashyap A, Atkinson R, Sniecinski I, Weiner LP et al. Peripheral blood stem cell transplantation in multiple sclerosis with busulfan and cyclophosphamide conditioning: report of toxicity and immunological monitoring. Biol Blood Marrow Transplant 2000; 6: 563–575.
Saccardi R, Mancardi GL, Solari A, Bosi A, Bruzzi P, Di Bartolomeo P et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood 2005; 105: 2601–2607.
Shevchenko YL, Novik AA, Kuznetsov AN, Afanasiev BV, Lisukov IA, Kozlov VA et al. High-dose immunosuppressive therapy with autologous hematopoietic stem cell transplantation as a treatment option in multiple sclerosis. Exp Hematol 2008; 36: 922–928.
Su L, Xu J, Ji BX, Wan SG, Lu CY, Dong HQ et al. Autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Int J Hematol 2006; 84: 276–281.
Xu J, Ji BX, Su L, Dong HQ, Sun XJ, Liu CY . Clinical outcomes after autologous haematopoietic stem cell transplantation in patients with progressive multiple sclerosis. Chin Med J (Engl) 2006; 119: 1851–1855.
Saccardi R, Kozak T, Bocelli-Tyndall C, Fassas A, Kazis A, Havrdova E et al. Autoimmune Diseases Working Party of EBMT. Autologous stem cell transplantation for progressive multiple sclerosis: update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Mult Scler 2006; 12: 814–823.
Burt RK, Cohen BA, Russell E, Spero K, Joshi A, Oyama Y et al. Hematopoietic stem cell transplantation for progressive multiple sclerosis: failure of a total body irradiation-based conditioning regimen to prevent disease progression in patients with high disability scores. Blood 2003; 102: 2373–2378.
Nash RA, Bowen JD, McSweeney PA, Pavletic SZ, Maravilla KR, Park MS et al. High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood 2003; 102: 2364–2372.
CAMMS223 Trial Investigators Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008; 359: 1786–1801.
Krishnan C, Kaplin AI, Brodsky RA, Drachman DB, Jones RJ, Pham DL et al. Reduction of disease activity and disability with high-dose cyclophosphamide in patients with aggressive multiple sclerosis. Arch Neurol 2008; 65: 1044–1051.
Roxburgh RH, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, Vukusic S et al. Multiple sclerosis severity score: using disability and disease duration to rate disease severity. Neurology 2005; 64: 1144–1151.
Snowden JA, Biggs JC, Milliken ST, Fuller A, Staniforth D, Passuello F et al. A randomised, blinded, placebo-controlled, dose escalation study of the tolerability and efficacy of filgrastim for haemopoietic stem cell mobilisation in patients with severe active rheumatoid arthritis. Bone Marrow Transplant 1998; 22: 1035–1041.
Openshaw H, Stuve O, Antel JP, Nash R, Lund BT, Weiner LP et al. Multiple sclerosis flares associated with recombinant granulocyte colony-stimulating factor. Neurology 2000; 54: 2147–2150.
Statkute L, Verda L, Oyama Y, Traynor A, Villa M, Shook T et al. Mobilization, harvesting and selection of peripheral blood stem cells in patients with autoimmune diseases undergoing autologous hematopoietic stem cell transplantation. Bone Marrow Transplant 2007; 39: 317–329.
Snir O, Lavie G, Achiron A, Bank I, Ben-Aharon T, Sredni B et al. G-CSF enhances the adhesion of encephalitogenic T cells to extracellular matrix components: a possible mechanism for exacerbation of multiple sclerosis. J Neuroimmunol 2006; 172: 145–155.
Burt RK, Fassas A, Snowden J, van Laar JM, Kozak T, Wulffraat NM et al. Collection of hematopoietic stem cells from patients with autoimmune diseases. Bone Marrow Transplant 2001; 28: 1–12.
Bonig H, Wundes A, Chang KH, Lucas S, Papayannopoulou T . Increased numbers of circulating hematopoietic stem/progenitor cells are chronically maintained in patients treated with the CD49d blocking antibody natalizumab. Blood 2008; 111: 3439–3441.
Zohren F, Toutzaris D, Klärner V, Hartung HP, Kieseier B, Haas R . The monoclonal anti-VLA-4 antibody natalizumab mobilizes CD34+ hematopoietic progenitor cells in humans. Blood 2008; 111: 3893–3895.
Uy GL, Rettig MP, Cashen AF . Plerixafor, a CXCR4 antagonist for the mobilization of hematopoietic stem cells. Expert Opin Biol Ther 2008; 8: 1797–1804.
Cogle CR, Yachnis AT, Laywell ED, Zander DS, Wingard JR, Steindler DA et al. Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet 2004; 363: 1432–1437.
Mezey E, Key S, Vogelsang G, Szalayova I, Lange GD, Crain B . Transplanted bone marrow generates new neurons in human brains. Proc Natl Acad Sci USA 2003; 100: 1364–1369.
Lu JQ, Storek J, Metz L, Yong VW, Stevens AM, Nash RA et al. Continued disease activity in a patient with multiple sclerosis after allogeneic hematopoietic cell transplantation. Arch Neurol 2009; 66: 116–120.
Rice CM, Mallam EA, Whone AL, Walsh P, Brooks DJ, Kane N et al. Safety and feasibility of autologous bone marrow cellular therapy in relapsing-progressive multiple sclerosis. Clin Pharmacol Ther 2010; 87: 679–685.
Haller MJ, Wasserfall CH, McGrail KM, Cintron M, Brusko TM, Wingard JR et al. Autologous umbilical cord blood transfusion in very young children with type 1 diabetes. Diabetes Care 2009; 32: 2041–2046.
Berisso GA, van Lint MT, Bacigalupo A, Marmont AM . Adoptive autoimmune hyperthyroidism following allogeneic stem cell transplantation from an HLA-identical sibling with Graves’ disease. Bone Marrow Transplant 1999; 23: 1091–1092.
Samijn JP, te Boekhorst PA, Mondria T, van Doorn PA, Flach HZ, van der Mech FG et al. Intense T cell depletion followed by autologous bone marrow transplantation for severe multiple sclerosis. J Neurol Neurosurg Psychiatry 2006; 77: 46–50.
Sun W, Popat U, Hutton G, Zang YC, Krance R, Carrum G et al. Characteristics of T-cell receptor repertoire and myelin-reactive T cells reconstituted from autologous haematopoietic stem-cell grafts in multiple sclerosis. Brain 2004; 127: 996–1008.
Atkins H, Freedman M . Immune ablation followed by autologous hematopoietic stem cell transplantation for the treatment of poor prognosis multiple sclerosis. Methods Mol Biol 2009; 549: 231–246.
Farge D, Labopin M, Tyndall A, Fassas A, Mancardi GL, Van Laar J et al. Autologous hematopoietic stem cell transplantation (HSCT) for autoimmune diseases: an observational study on 12 years of experience from the European Group for Blood and Marrow Transplantation (EBMT) working party on autoimmune diseases. Haematologica 2010; 95: 284–292.
Stem cell therapy for patients with multiple sclerosis failing interferon A randomized study. Available from http://www.clinicaltrials.gov/ct21/show/record/NCT00273364.
ASTI MS (Autologous Stem cell Transplantation International Multiple Sclerosis). Available from: http://www.astims.org.
HALT-MS (High Dose Immunosuppression and Autologous Stem Cell Transplantation for Poor Prognosis Multiple Sclerosis). Available from: http://www.halt-ms.org and www.clinicaltrials.gov/ct21/show/record/NCT00288626.
Nash RA, Dansey R, Storek J, Georges GE, Bowen JD, Holmberg LA et al. Epstein-Barr virus-associated posttransplantation lymphoproliferative disorder after high-dose immunosuppressive therapy and autologous CD34-selected hematopoietic stem cell transplantation for severe autoimmune diseases. Biol Blood Marrow Transplant 2003; 9: 583–591.
Oyama Y, Cohen B, Traynor A, Brush M, Rodriguez J, Burt RK . Engraftment syndrome: a common cause for rash and fever following autologous hematopoietic stem cell transplantation for multiple sclerosis. Bone Marrow Transplant 2002; 29: 81–85.
Loh Y, Oyama Y, Statkute L, Yaung K, Gonda E, Barr W et al. Development of a secondary autoimmune disorder after hematopoietic stem cell transplantation for autoimmune diseases: role of conditioning regimen used. Blood 2007; 109: 2643–2548.
Griffith LM, Pavletic SZ, Tyndall A, Bredeson CN, Bowen JD, Childs RW et al. Feasibility of allogeneic hematopoietic stem cell transplantation for autoimmune disease: position statement from a National Institute of Allergy and Infectious Diseases and National Cancer Institute-Sponsored International Workshop, Bethesda, MD, March 12 and 13, 2005. Biol Blood Marrow Transplant 2005; 11: 862–870.
van Gelder M, van Bekkum DW . Treatment of relapsing experimental autoimmune encephalomyelitis in rats with allogeneic bone marrow transplantation from a resistant strain. Bone Marrow Transplant 1995; 16: 343–351.
Van Wijmeersch B, Sprangers B, Rutgeerts O, Lenaerts C, Landuyt W, Waer M et al. Allogeneic bone marrow transplantation in models of experimental autoimmune encephalomyelitis: evidence for a graft-versus-autoimmunity effect. Biol Blood Marrow Transplant 2007; 13: 627–637.
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008; 371: 1579–1586.
Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia 2009; 57: 1192–1203.
Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005; 106: 1755–1761.
Rafei M, Birman E, Forner K, Galipeau J . Allogeneic mesenchymal stem cells for treatment of experimental autoimmune encephalomyelitis. Mol Ther 2009; 17: 1799–1803.
Liang J, Zhang H, Hua B, Wang H, Wang J, Han Z et al. Allogeneic mesenchymal stem cells transplantation in treatment of multiple sclerosis. Mult Scler 2009; 15: 644–646.
Mohyeddin Bonab M, Yazdanbakhsh S, Lotfi J, Alimoghaddom K, Talebian F, Hooshmand F et al. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol 2007; 4: 50–57.
Tappenden P, Saccardi R, Confavreux C, Sharrack B, Muraro PA, Mancardi GL et al. Autologous haematopoietic stem cell transplantation for secondary progressive multiple sclerosis: an exploratory cost-effectiveness analysis. Bone Marrow Transplant 2010; 45: 1014–1021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Atkins, H. Hematopoietic SCT for the treatment of multiple sclerosis. Bone Marrow Transplant 45, 1671–1681 (2010). https://doi.org/10.1038/bmt.2010.168
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2010.168
Keywords
This article is cited by
-
Stammzelltransplantation bei Multipler Sklerose
Der Nervenarzt (2015)
-
Hematopoietic Stem Cell Therapy for Multiple Sclerosis: Top 10 Lessons Learned
Neurotherapeutics (2013)
-
Increased covalent conjugation of a model antigen to poly(lactic acid)-g-maleic anhydride nanoparticles compared to bare poly(lactic acid) nanoparticles
Colloid and Polymer Science (2013)
-
Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation
Bone Marrow Transplantation (2012)