Abstract
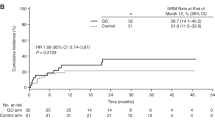
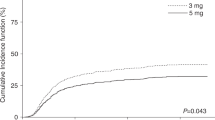
BU–CY is the established non-TBI-based myeloablative conditioning regimen for allogeneic hematopoietic SCT. However, liver toxicity and hepatic veno-occlusive disease (VOD) are frequent life-threatening complications. Pharmacological considerations suggest that BU can trigger toxicity of subsequent CY. Recent animal data confirmed this hypothesis. Less liver toxicity and better outcomes were observed when mice were treated with the reversed order of CY and BU. We analyzed in this study liver toxicity and outcome in patients receiving BU–CY (16 patients) or CY–BU (59 patients). Liver function differed significantly with higher levels of liver function tests between day +10 and +30, and a higher cumulative incidence of VOD in the BU–CY cohort (2/16 (12.5%) vs 0/59 (0%), P=0.006). TRM was significantly higher in patients receiving BU–CY (cumulative incidence BU–CY 45%, CY–BU 17%, P=0.02), without yet translating into a significant survival difference (incidence for survival: BU–CY 38%, CY–BU 63%; hazard ratio 1.19 for BU–CY, 95% confidence interval 0.29–4.82, P=0.80). Rates of engraftment and relapse were not different. These data support the concepts derived from animal models in favor of CY–BU compared with traditional BU–CY and form the basis for prospective controlled comparisons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Socie G, Clift RA, Blaise D, Devergie A, Ringden O, Martin PJ et al. Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia: long-term follow-up of 4 randomized studies. Blood 2001; 98: 3569–3574.
Hassan M . The role of busulfan in bone marrow transplantation. Med Oncol 1999; 16: 166–176.
Clift RA, Buckner CD, Thomas ED, Bensinger WI, Bowden R, Bryant E et al. Marrow transplantation for chronic myeloid leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood 1994; 84: 2036–2043.
Brodsky R, Topolsky D, Crilley P, Bulova S, Brodsky I . Frequency of veno-occlusive disease of the liver in bone marrow transplantation with a modified busulfan/cyclophosphamide preparative regimen. Am J Clin Oncol 1990; 13: 221–225.
Vassal G, Hartmann O, Benhamou E . Busulfan and veno-occlusive disease of the liver. Ann Intern Med 1990; 112: 881.
Ljungman P, Hassan M, Bekassy AN, Ringden O, Oberg G . High busulfan concentrations are associated with increased transplant-related mortality in allogeneic bone marrow transplant patients. Bone Marrow Transplant 1997; 20: 909–913.
Nilsson C, Aschan J, Hentschke P, Ringden O, Ljungman P, Hassan M . The effect of metronidazole on busulfan pharmacokinetics in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 2003; 31: 429–435.
Buggia I, Zecca M, Alessandrino EP, Locatelli F, Rosti G, Bosi A et al. Itraconazole can increase systemic exposure to busulfan in patients given bone marrow transplantation. GITMO (Gruppo Italiano Trapianto di Midollo Osseo). Anticancer Res 1996; 16 (4A): 2083–2088.
Meresse V, Hartmann O, Vassal G, Benhamou E, Valteau-Couanet D, Brugieres L et al. Risk factors for hepatic veno-occlusive disease after high-dose busulfan-containing regimens followed by autologous bone marrow transplantation: a study in 136 children. Bone Marrow Transplant 1992; 10: 135–141.
McDonald GB, Slattery JT, Bouvier ME, Ren S, Batchelder AL, Kalhorn TF et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood 2003; 101: 2043–2048.
McCune JS, Batchelder A, Deeg HJ, Gooley T, Cole S, Phillips B et al. Cyclophosphamide following targeted oral busulfan as conditioning for hematopoietic cell transplantation: pharmacokinetics, liver toxicity, and mortality. Biol Blood Marrow Transplant 2007; 13: 853–862.
Hassan M, Ljungman P, Ringden O, Hassan Z, Oberg G, Nilsson C et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant 2000; 25: 915–924.
DeLeve LD, Wang X . Role of oxidative stress and glutathione in busulfan toxicity in cultured murine hepatocytes. Pharmacology 2000; 60: 143–154.
Hassan Z, Hellstrom-Lindberg E, Alsadi S, Edgren M, Hagglund H, Hassan M . The effect of modulation of glutathione cellular content on busulphan-induced cytotoxicity on hematopoietic cells in vitro and in vivo. Bone Marrow Transplant 2002; 30: 141–147.
Nilsson C, Forsman J, Hassan Z, Abedi-Valugerdi M, O’Connor C, Concha H et al. Effect of altering administration order of busulphan and cyclophosphamide on the myeloablative and immunosuppressive properties of the conditioning regimen in mice. Exp Hematol 2005; 33: 380–387.
Ferrara JL . The cytokine modulation of acute graft-versus-host disease. Bone Marrow Transplant 1998; 21 (Suppl 3): S13–S15.
Via CS, Finkelman FD . Critical role of interleukin-2 in the development of acute graft-versus-host disease. Int Immunol 1993; 5: 565–572.
Hill GR, Teshima T, Rebel VI, Krijanovski OI, Cooke KR, Brinson YS et al. The p55 TNF-alpha receptor plays a critical role in T cell alloreactivity. J Immunol 2000; 164: 656–663.
Sadeghi B, Jansson M, Hassan Z, Mints M, Hagglund H, Abedi-Valugerdi M et al. The effect of administration order of BU and CY on engraftment and toxicity in HSCT mouse model. Bone Marrow Transplant 2008; 41: 895–904.
Stussi G, Halter J, Tichelli A, Meyer-Monard S, Buser AS, Arber C et al. Double allogeneic hematopoietic SCT as a rescue therapy for poor-risk hematological malignancies. Bone Marrow Transplant 2010; 45: 103–109.
Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de Witte T et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer 2009; 115: 4715–4726.
Cantoni N, Weisser M, Buser A, Arber C, Stern M, Heim D et al. Infection prevention strategies in a stem cell transplant unit: impact of change of care in isolation practice and routine use of high dose intravenous immunoglobulins on infectious complications and transplant related mortality. Eur J Haematol 2009; 83: 130–138.
Heim D, Passweg J, Gregor M, Buser A, Theocharides A, Arber C et al. Patient and product factors affecting platelet transfusion results. Transfusion 2008; 48: 681–687.
Bearman SI . The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood 1995; 85: 3005–3020.
Tsakiris DA, Tichelli A . Thrombotic complications after haematopoietic stem cell transplantation: early and late effects. Best Pract Res Clin Haematol 2009; 22: 137–145.
Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med 2001; 344: 1815–1822.
Thomas E, Storb R, Clift RA, Fefer A, Johnson FL, Neiman PE et al. Bone-marrow transplantation (first of two parts). N Engl J Med 1975; 292: 832–843.
Deeg HJ, Storb R . Graft-versus-host disease: pathophysiological and clinical aspects. Annu Rev Med 1984; 35: 11–24.
Siekmann L, Bonora R, Burtis CA, Ceriotti F, Clerc-Renaud P, Ferard G et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. Part 1. The concept of reference procedures for the measurement of catalytic activity concentrations of enzymes. Clin Chem Lab Med 2002; 40: 631–634.
Scharf SJ, Smith AG, Hansen JA, McFarland C, Erlich HA . Quantitative determination of bone marrow transplant engraftment using fluorescent polymerase chain reaction primers for human identity markers. Blood 1995; 85: 1954–1963.
Wicki A, Malik N, Gratwohl A, Tichelli A, Meyer-Monard S, Muller H . Determination of the donor:host blood cell ratio after haematological stem cell transplant by means of semiquantitative detection of short tandem repeat polymorphisms. Swiss Med Wkly 2002; 132: 288–295.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AG and NC received research support from Pierre Fabre SA.
Rights and permissions
About this article
Cite this article
Cantoni, N., Gerull, S., Heim, D. et al. Order of application and liver toxicity in patients given BU and CY containing conditioning regimens for allogeneic hematopoietic SCT. Bone Marrow Transplant 46, 344–349 (2011). https://doi.org/10.1038/bmt.2010.137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2010.137
Keywords
This article is cited by
-
Effect of pharmacokinetics and pharmacogenomics in adults with allogeneic hematopoietic cell transplantation conditioned with Busulfan
Bone Marrow Transplantation (2023)
-
Does the order of busulfan and cyclophosphamide affect allogeneic stem cell transplantation related liver toxicity?
Annals of Hematology (2021)
-
Busulfan-cyclophosphamide versus cyclophosphamide-busulfan as conditioning regimen before allogeneic hematopoietic cell transplantation: a prospective randomized trial
Annals of Hematology (2021)
-
Busulfan–Melphalan followed by autologous stem cell transplantation in patients with high-risk neuroblastoma or Ewing sarcoma: an exposed–unexposed study evaluating the clinical impact of the order of drug administration
Bone Marrow Transplantation (2016)
-
A modified busulfan and cyclophosphamide preparative regimen for allogeneic transplantation in myeloid malignancies
International Journal of Clinical Pharmacy (2015)