Abstract
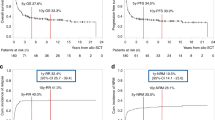
The role of autologous hematopoietic SCT (autoHSCT) in the treatment of high-risk (HR) adult ALL is controversial. In this study, we retrospectively analyzed the results of autoHSCT according to the status of minimal residual disease (MRD) at transplantation, as a joint analysis of the European Study Group for Adult ALL (EWALL). Data on 123 recipients of autoHSCT, aged 31 (16–59) years, with B-lineage (n=77) or T-lineage (n=46) ALL were included. In a cohort of Ph-negative ALL, the probability of leukemia-free survival at 5 years was higher for patients with MRD <0.1% compared with those with MRD ⩾0.1% (57 vs 17%, P=0.0002). The difference was significant for T-lineage ALL (62 vs 8%, P=0.001), and a tendency was observed for B-lineage ALL (54 vs 26%, P=0.17). In a multivariate analysis, adjusted for other potential prognostic factors, high MRD level remained the only independent factor associated with increased risk of failure (risk ratio, 2.8; P=0.0005). We conclude that MRD determines the outcome of autoHSCT in HR adult ALL. Our results suggest the need to reevaluate the role of this treatment option in prospective trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rowe JM, Goldstone AH . How I treat acute lymphocytic leukemia in adults. Blood 2007; 110: 2268–2275.
Hoelzer D, Thiel E, Löffler H, Büchner T, Ganser A, Heil G et al. Prognostic factors in a multicenter study for treatment of acute lymphoblastic leukemia in adults. Blood 1988; 71: 123–131.
Hahn T, Wall D, Camitta B, Davies S, Dillon H, Gaynon P et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute lymphoblastic leukemia in adults: an evidence-based review. Biol Blood Marrow Transplant 2006; 12: 1–30.
Willemze R, Labar B . Post-remission treatment for adult patients with acute lymphoblastic leukemia in first remission: is there a role for autologous stem cell transplantation? Semin Hematol 2007; 44: 267–273.
Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol 2004; 22: 4075–4086.
Ribera JM, Oriol A, Bethencourt C, Parody R, Hernández-Rivas JM, Moreno MJ et al. Comparison of intensive chemotherapy, allogeneic or autologous stem cell transplantation as post-remission treatment for adult patients with high-risk acute lymphoblastic leukemia. Results of the PETHEMA ALL-93 trial. Haematologica 2005; 90: 1346–1356.
Labar B, Suciu S, Zittoun R, Muus P, Marie JP, Fillet G et al. Allogeneic stem cell transplantation in acute lymphoblastic leukemia and non-Hodgkin's lymphoma for patients <or=50 years old in first complete remission: results of the EORTC ALL-3 trial. Haematologica 2004; 89: 809–817.
Goldstone AH, Richards SM, Lazarus HM, Goldstone AH, Richards SM, Lazarus HM et al. In adults with standard-risk acute lymphoblastic leukemia (ALL) the greatest benefit is achieved from a matched sibling allogeneic transplant in first complete remission (CR) and an autologous transplant is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the international ALL trial (MRC UKALL XII/ECOG E2993). Blood 2008; 111: 1827–1833.
Dhédin N, Dombret H, Thomas X, Lhéritier V, Boiron JM, Rigal-Huguet F et al. Autologous stem cell transplantation in adults with acute lymphoblastic leukemia in first complete remission: analysis of the LALA-85, -87 and -94 trials. Leukemia 2006; 20: 336–344.
Hołowiecki J, Giebel S, Krzemień S, Krawczyk-Kuliś M, Jagoda K, Kopera M et al. G-CSF administered in time-sequenced setting during remission induction and consolidation therapy of adult acute lymphoblastic leukemia has beneficial influence on early recovery and possibly improves long-term outcome: a randomized multicenter study. Leuk Lymphoma 2002; 43: 315–325.
Holowiecki J, Krawczyk-Kulis M, Giebel S, Jagoda K, Stella-Holowiecka B, Piatkowska-Jakubas B et al. Status of minimal residual disease after induction predicts outcome in both standard and high risk Ph-negative adult acute lymphoblastic leukemia. The Polish Adult Leukemia Group ALL 4-2002 MRD study. Br J Haematol 2008; 142: 227–237.
Brüggemann M, Raff T, Flohr T, Gökbuget N, Nakao M, Droese J et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood 2006; 107: 1116–1123.
Attal M, Blaise D, Marit G, Payen C, Michallet M, Vernant JP et al. Consolidation treatment of adult acute lymphoblastic leukemia: a prospective, randomized trial comparing allogeneic versus autologous bone marrow transplantation and testing the impact of recombinant interleukin-2 after autologous bone marrow transplantation. BGMT Group. Blood 1995; 86: 1619–1628.
de Labarthe A, Rousselot P, Huguet-Rigal F, Delabesse E, Witz F, Maury S et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study. Blood 2007; 109: 1408–1413.
Ribera JM, Oriol A, Bethencourt C, Parody R, Hernández-Rivas JM, Moreno MJ et al. Comparison of intensive chemotherapy, allogeneic or autologous stem cell transplantation as post-remission treatment for adult patients with high-risk acute lymphoblastic leukemia. Results of the PETHEMA ALL-93 trial. Haematologica 2005; 90: 1346–1356.
Mancini M, Scappaticci D, Cimino G, Nanni M, Derme V, Elia L et al. A comprehensive genetic classification of adult acute lymphoblastic leukemia (ALL): analysis of the GIMEMA 0496 protocol. Blood 2005; 105: 3434–3441.
Lucio P, Gaipa G, van Lochem EG, van Wering ER, Porwit-MacDonald A, Faria T et al. BIOMED-1 concerted action report: flow cytometric immunophenotyping of precursor B-ALL with standarized triple-stainings. Leukemia 2001; 15: 1185–1192.
Porwit-MacDonald A, Bjorklund E, Lucio P, van Lochem EG, Mazur J, Parreira A et al. BIOMED-1 Concerted Action report: Flow cytometric characterisation of CD7 cell subsets in normal bone marrow as a basis for the diagnosis and follow-up of T cell acute lymphoblastic leukemia (T-ALL). Leukemia 2000; 14: 816–825.
Lucio P, Parreira A, van den Beemd MW, van Lochem EG, van Wering ER, Baars E et al. Flow cytometric analysis of normal B cell differentiation: a frame of reference for the detection of minimal residual disease in precursor-B-ALL. Leukemia 1999; 13: 419–427.
Ciudad J, Orfao A, Vidriales B, Macedo A, Martínez A, González M et al. Immunophenotypic analysis of CD19+ precursors in normal human adult bone marrow: implications for minimal residual disease detection. Haematologica 1998; 83: 1069–1075.
Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—a Europe Against Cancer program. Leukemia 2003; 17: 2318–2357.
Kaplan EL, Meier P . Nonparametric estimations from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Cox DR . Regression models and life tables. J Royal Soc B 1972; 34: 187–220.
Kiehl MG, Kraut L, Schwerdtfeger R, Hertenstein B, Remberger M, Kroeger N et al. Outcome of allogeneic hematopoietic stem-cell transplantation in adult patients with acute lymphoblastic leukemia: no difference in related compared with unrelated transplant in first complete remission. J Clin Oncol 2004; 22: 2816–2825.
Zaliova M, Fronkova E, Krejcikova K, Muzikova K, Mejstrikova E, Stary J et al. Quantification of fusion transcript reveals a subgroup with distinct biological properties and predicts relapse in BCR/ABL-positive ALL: implications for residual disease monitoring. Leukemia 2009; 23: 944–951.
Szczepański T . Why and how to quantify minimal residual disease in acute lymphoblastic leukemia? Leukemia 2007; 21: 622–626.
Willemse MJ, Seriu T, Hettinger K, d'Aniello E, Hop WC, Panzer-Grümayer ER et al. Detection of minimal residual disease identifies differences in treatment response between T-ALL and precursor B-ALL. Blood 2002; 99: 4386–4393.
Martin H, Atta J, Bruecher J, Elsner S, Schardt C, Stadler M et al. In patients with BCR-ABL-positive ALL in CR peripheral blood contains less residual disease than bone marrow: implications for autologous BMT. Ann Hematol 1994; 68: 85–87.
Acknowledgements
The contributions of the scientists involved in MRD assessment, Krystyna Jagoda, PhD, Agnieszka Balana-Nowak, PhD, Monika Brüggemann PhD, and Thorsten Raff, PhD, are acknowledged.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Giebel, S., Stella-Holowiecka, B., Krawczyk-Kulis, M. et al. Status of minimal residual disease determines outcome of autologous hematopoietic SCT in adult ALL. Bone Marrow Transplant 45, 1095–1101 (2010). https://doi.org/10.1038/bmt.2009.308
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2009.308
Keywords
This article is cited by
-
Safety and efficacy of CD22 and CD19 CAR-T bridging auto-HSCT as consolidation therapy for AYA and adult B-ALL
Blood Cancer Journal (2023)
-
Autologous versus allogeneic hematopoietic cell transplantation for older patients with acute lymphoblastic leukemia. An analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation
Bone Marrow Transplantation (2023)
-
Propensity score matching/reweighting analysis comparing autologous and allogeneic stem cell transplantation for B-lineage acute lymphoblastic leukemia
International Journal of Hematology (2022)
-
Evolving therapy of adult acute lymphoblastic leukemia: state-of-the-art treatment and future directions
Journal of Hematology & Oncology (2020)
-
Hematopoietic stem cell transplantation for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first remission: a position statement of the European Working Group for Adult Acute Lymphoblastic Leukemia (EWALL) and the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT)
Bone Marrow Transplantation (2019)