Abstract
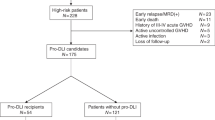
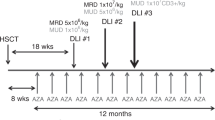
Donor lymphocyte infusion (DLI) exerts a GVL effect, but its use is limited by a high incidence of GVHD. We retrospectively evaluated the efficacy of administering short-term immunosuppressive agents for prophylaxis against DLI-associated acute GVHD, and its influence on the GVL effect. Seventy patients with leukaemia received G-CSF primed DLI after HLA-identical sibling haematopoietic stem cell transplantation (HSCT) for treatment or prophylaxis against leukaemia relapse. Short-term immunosuppressive agents were given to 54 patients for prophylaxis against DLI-associated acute GVHD. Seventeen patients experienced acute GVHD; 30 patients developed chronic GVHD; and no GVHD-related death was observed. A significant difference was observed between the group that did not receive prophylaxis against GVHD or received prophylaxis for less than 2 weeks and the group that received prophylaxis for over 2 weeks (CsA or MTX at 10 mg/week) with regard to the incidence of DLI-associated acute GVHD (14/28 vs 3/42, P=0.000); no difference was observed in the relapse rate for prophylactic DLI patients between the two groups (4/10 vs 12/29). Using immunosuppressive agents for 2–4 weeks may reduce DLI-associated acute GVHD without influencing relapse and survival after G-CSF-primed DLI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Grigg AP, Szer J, Beresford J, Dodds A, Bradstock K, Durrant S et al. Factors affecting the outcome of allogeneic bone marrow transplantation for adult patients with refractory or relapsed acute leukemia. Br J Haematol 1999; 107: 409–418.
Barrett AJ, Locatelli F, Treleaven JG, Gratwohl A, Szydło R, Zwaan FE . Second transplants for leukemic relapse after bone marrow transplantation: high early mortility but favorable effect of chronic GVHD on continued remission. Br J Haematol 1991; 79: 567–574.
Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. European Group for Blood and Marrow Transplantation Working party Chronic Leukemia. Blood 1995; 86: 2041–2050.
Collins Jr RH, Goldstein S, Giralt S, Levine J, Porter D, Drobyski W et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant 2000; 26: 511–516.
Alessandrino EP, Bernasconi P, Caldera D, Colombo A, Malcovati L, Martinelli G et al. Chemotherapy and donor peripheral blood progenitor cells for acute leukemia in early relapse after allogeneic bone marrow transplantation. Bone Marrow Transplant 1999; 23: 607–612.
Sohn SK, Jung JT, Kim DH, Lee NY, Seo KW, Chae YS et al. Prophylactic growth factor-primed donor lymphocyte infusion using cells reserved at the time of transplantation after allogeneic peripheral blood stem cell transplantation in patients with high-risk hematologic malignancies. Cancer 2002; 94: 18–24.
Huang X, Guo N, Ren H, Zhang Y, Gao Z, Lu D . An improved anti-leukemic effect achieved with donor progenitor cell infusion for relapse patients after allogeneic bone marrow transplantation. Chin Med J 2003; 116: 736–741.
Huang XJ, Liu DH, Xu LP, Chen H, Han W, Liu KY et al. Prophylactic infusion of donor granulocyte colony stimulating factor mobilized peripheral blood progenitor cells after allogeneic hematological stem cell transplantation in patients with high-risk leukemia. Leukemia 2006; 20: 365–368.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W . Donor lymphocyte infusion for the treatment of leukemia relapse after HLA-mismatched/haploidentical T-cell-replete hematopoietic stem cell transplantation. Haematologica 2007; 92: 414–417.
Huang XJ, Wang Y, Liu DH, Xu LP, Chen H, Chen YH et al. Modified donor lymphocyte infusion (DLI) for the prophylaxis of leukemia relapse after hematopoietic stem cell transplantation in patients with advanced leukemia—feasibility and safety study. J Clin Immunol 2008; 28: 390–397.
Devergie A . Graft vs host disease. In: Apperley J, Carreras E, Gluckman E, Gratwohl A, Masszi T (eds). Haematopoietic Stem Cell Transplantation: The EBMT Handbook, Rev edn. European School of Hematology: Paris, France, 2004, pp 168–169.
Weisdorf D . GVHD—The Nuts and Bolts. In: Gewirtz AM, Winter JN, Zuckerman K (eds). American Society of Hematology Education Program Book. Washington, DC, USA, 2007, pp 62–67.
Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 2006; 108: 1092–1099.
Slavin S, Naparastek E, Nagler A, Ackerstein A, Kapelushnik J, Or R . Allogeneic cell therapy for relapsed leukemia after bone marrow transplantation with donor peripheral blood lymphocytes. Exp Hematol 1995; 23: 1553–1562.
Huang XJ, Chang YJ, Zhao XY . Maintaining hyporesponsiveness and polarization potential of T cells after in vitro mixture of G-CSF mobilized peripheral blood grafts and G-CSF primed bone marrow grafts in different proportions. Transplant immunol 2007; 17: 193–197.
Huang XJ, Chang YJ, Zhao XY . In vivo induction of T-cell hyporesponsiveness and alteration of immunological cells of bone marrow grafts using granulocyte colony-stimulating factor. Haematologica 2004; 89: 1517–1524.
Chen SH, Li X, Huang XJ . Effect of recombinant granulocyte colony-stimulating factor on T-lymphocyte function and the mechanism of this effect. Int J Hematol 2004; 79: 178–184.
Zeng D, Dejbakhsh-Jones S, Strorber S . Granulocyte colony stimulating factor reduces the capacity of blood mononuclear cells to induce graft-versus-host disease: impact on blood progenitor cell transplantation. Blood 1997; 90: 453–463.
Dazzi F, Szydlo RM, Cross NC, Craddock C, Kaeda J, Kanfer E et al. Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood 2000; 96: 2712–2716.
Weisser M, Tischer J, Schnittger S, Schoch C, Ledderose G, Kolb HJ . A comparison of donor lymphocyte infusions or imatinib mesylate for patients with chronic myelogenous leukemia who have relapsed after allogeneic stem cell transplantation. Haematologica 2006; 91: 663–666.
Collins RH, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R et al. Donor leukocyte infusion in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol 1997; 15: 433–444.
Kim JG, Sohn SK, Kim DH, Lee NY, Suh JS, Lee KS et al. A pilot study of cytoreductive chemotherapy combined with infusion of additional peripheral blood stem cells reserved at time of harvest for transplantation in case of relapsed hematologic malignancies after allogeneic peripheral blood stem cell transplant. Bone marrow transplant 2004; 33: 231–236.
Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT acute leukemia working party. J Clin Oncol 2007; 25: 4938–4945.
Acknowledgements
We thank Enpapers Team for critically reviewing this paper in English. This work was supported by the National Outstanding Young Scientist's Foundation of China (Grant no. 30725038), Hi-tech Research and Development Program of China (Grant no. 2006AA02Z4A0), Program for Innovative Research Team in University (Grant no. IRT 0702), Scientific Research Fund for Capital Medicine Development (Grant no. 2006-1010) and Leading Program of Clinical Faculty accredited by the Ministry of Health of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, XJ., Wang, Y., Liu, DH. et al. Administration of short-term immunosuppressive agents after DLI reduces the incidence of DLI-associated acute GVHD without influencing the GVL effect. Bone Marrow Transplant 44, 309–316 (2009). https://doi.org/10.1038/bmt.2009.26
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2009.26
Keywords
This article is cited by
-
Evolution of cell therapy for renal cell carcinoma
Molecular Cancer (2024)
-
TET2 mutations contribute to adverse prognosis in acute myeloid leukemia (AML): results from a comprehensive analysis of 502 AML cases and the Beat AML public database
Clinical and Experimental Medicine (2024)
-
Full donor chimerism without graft-versus-host disease: the key factor for maximum benefit of pre-emptive donor lymphocyte infusions (pDLI)
Bone Marrow Transplantation (2020)
-
Minimal residual disease-directed immunotherapy for high-risk myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation
Frontiers of Medicine (2019)
-
Impact of pre-transplantation minimal residual disease determined by multiparameter flow cytometry on the outcome of AML patients with FLT3-ITD after allogeneic stem cell transplantation
Annals of Hematology (2018)