Abstract
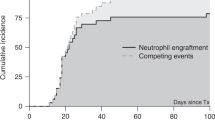
The precise effects of CD34+ cell dose on the outcome of allogeneic transplantation for aplastic anaemia (AA) are not known. Previous studies have used the total mononuclear cell count to quantify stem cell dose. We evaluated the effects of CD34+ cell dose on the clinical and haematological end points of transplantation. The transplant variables and outcome parameters on 46 patients with acquired AA were assessed by comparing low vs high CD34+ cell doses. Infusion of less than 2 × 106/kg of CD34+ cells was associated with an increased incidence of graft failures (P=0.03), higher incidence of bacterial infections (P=0.006) and a delay in the engraftment of neutrophils (P=0.046). The latter was found to be an effect of stem cell source (non-PBSC) rather than the CD34+ count. Other parameters, such as plt engraftment (P=0.63), red cell (P=0.94) and plt (P=0.31) transfusion independence, chimerism, acute and chronic GVHD (P=1.0) and OS (P=0.57), were not significantly influenced by the CD34+ cell dose. These findings are different to the published studies on the relevance of CD34+ cell dose in allogeneic transplantation for haematological cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Holyoake TL, Alcorn MJ . CD34+ positive haemopoietic cells: biology and clinical applications. Blood Rev 1994; 8: 113–124.
Siena S, Schiavo R, Pedrazzoli P, Carlo-Stella C . Therapeutic relevance of CD34 cell dose in blood cell transplantation for cancer therapy. J Clin Oncol 2000; 18: 1360–1377.
Singhal S, Powles R, Treleaven J, Kulkarni S, Sirohi B, Horton C et al. A low CD34+ cell dose results in higher mortality and poorer survival after blood or marrow stem cell transplantation from HLA-identical siblings: should 2 x 10(6) CD34+ cells/kg be considered the minimum threshold? Bone Marrow Transplant 2000; 26: 489–496.
Bittencourt H, Rocha V, Chevret S, Socie G, Esperou H, Devergie A et al. Association of CD34 cell dose with hematopoietic recovery, infections, and other outcomes after HLA-identical sibling bone marrow transplantation. Blood 2002; 99: 2726–2733.
Niederwieser D, Pepe M, Storb R, Loughran Jr TP, Longton G . Improvement in rejection, engraftment rate and survival without increase in graft-versus-host disease by high marrow cell dose in patients transplanted for aplastic anaemia. Br J Haematol 1988; 69: 23–28.
Bai LY, Chiou TJ, Liu JH, Yen CC, Wang WS, Yan MH et al. Hematopoietic stem cell transplantation for severe aplastic anemia: experience of an institute in Taiwan. Ann Hematol 2004; 83: 38–43.
McCann SR, Bacigalupo A, Gluckman E, Hinterberger W, Hows J, Ljungman P et al. Graft rejection and second bone marrow transplants for acquired aplastic anaemia: a report from the Aplastic Anaemia Working Party of the European Bone Marrow Transplant Group. Bone Marrow Transplant 1994; 13: 233–237.
Bacigalupo A, Hows J, Gluckman E, Nissen C, Marsh J, Van Lint MT et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol 1988; 70: 177–182.
Camitta BM, Thomas ED, Nathan DG, Santos G, Gordon-Smith EC, Gale RP et al. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood 1976; 48: 63–70.
Champlin RE, Horowitz MM, van Bekkum DW, Camitta BM, Elfenbein GE, Gale RP et al. Graft failure following bone marrow transplantation for severe aplastic anemia: risk factors and treatment results. Blood 1989; 73: 606–613.
Hoelle W, Beck JF, Dueckers G, Kreyenberg H, Lang P, Gruhn B et al. Clinical relevance of serial quantitative analysis of hematopoietic chimerism after allogeneic stem cell transplantation in children for severe aplastic anemia. Bone Marrow Transplant 2004; 33: 219–223.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Lee SJ, Vogelsang G, Flowers ME . Chronic graft-versus-host disease. Biol Blood Marrow Transplant 2003; 9: 215–233.
Morariu-Zamfir R, Rocha V, Devergie A, Socie G, Ribaud P, Esperou H et al. Influence of CD34(+) marrow cell dose on outcome of HLA-identical sibling allogeneic bone marrow transplants in patients with chronic myeloid leukaemia. Bone Marrow Transplant 2001; 27: 575–580.
Gomez-Almaguer D, Vela-Ojeda J, Jaime-Perez JC, Gutierrez-Aguirre CH, Cantu-Rodriguez OG, Sobrevilla-Calvo P et al. Allografting in patients with severe, refractory aplastic anemia using peripheral blood stem cells and a fludarabine-based conditioning regimen: the Mexican experience. Am J Hematol 2006; 81: 157–161.
Buchholz S, Dammann E, Koenecke CH, Stadler M, Franzke A, Blasczyk R et al. Allogeneic stem cell transplantation from related and unrelated donors for aplastic anemia in adults: a single-centre experience. Ann Hematol 2008; 87: 551–556.
Min CK, Kim DW, Lee JW, Han CW, Min WS, Kim CC . Hematopoietic stem cell transplantation for high-risk adult patients with severe aplastic anemia; reduction of graft failure by enhancing stem cell dose. Haematologica 2001; 86: 303–310.
Maury S, Balere-Appert ML, Chir Z, Boiron JM, Galambrun C, Yakouben K et al. Unrelated stem cell transplantation for severe acquired aplastic anemia: improved outcome in the era of high-resolution HLA matching between donor and recipient. Haematologica 2007; 92: 589–596.
Kojima S, Matsuyama T, Kato S, Kigasawa H, Kobayashi R, Kikuta A et al. Outcome of 154 patients with severe aplastic anemia who received transplants from unrelated donors: the Japan Marrow Donor Program. Blood 2002; 100: 799–803.
Locasciulli A, Oneto R, Bacigalupo A, Socie G, Korthof E, Bekassy A et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT). Haematologica 2007; 92: 11–18.
Ades L, Mary JY, Robin M, Ferry C, Porcher R, Esperou H et al. Long-term outcome after bone marrow transplantation for severe aplastic anemia. Blood 2004; 103: 2490–2497.
Bacigalupo A, Hows J, Gordon-Smith EC, Gluckman E, Van Lint MT, Congiu M et al. Bone marrow transplantation for severe aplastic anemia from donors other than HLA identical siblings: a report of the BMT Working Party. Bone Marrow Transplant 1988; 3: 531–535.
Resnick IB, Aker M, Shapira MY, Tsirigotis PD, Bitan M, Abdul-Hai A et al. Allogeneic stem cell transplantation for severe acquired aplastic anaemia using a fludarabine-based preparative regimen. Br J Haematol 2006; 133: 649–654.
Bacigalupo A, Oneto R, Bruno B, Socie G, Passweg J, Locasciulli A et al. Current results of bone marrow transplantation in patients with acquired severe aplastic anemia. Report of the European Group for Blood and Marrow transplantation. On behalf of the Working Party on Severe Aplastic Anemia of the European Group for Blood and Marrow Transplantation. Acta Haematol 2000; 103: 19–25.
Heimfeld S . Bone marrow transplantation: how important is CD34 cell dose in HLA-identical stem cell transplantation? Leukemia 2003; 17: 856–858.
Mielcarek M, Martin PJ, Heimfeld S, Storb R, Torok-Storb B et al. CD34 cell dose and chronic graft-versus-host disease after human leukocyte antigen-matched sibling hematopoietic stem cell transplantation. Leuk Lymphoma 2004; 45: 27–34.
Schrezenmeier H, Passweg JR, Marsh JCW, Bacigalupo A, Bredeson CN, Bullorsky E et al. Worse outcome and more chronic GVHD with peripheral blood progenitor cells than bone marrow in HLA-matched sibling donor transplants for young patients with severe acquired aplastic anemia. Blood 2007; 110: 1397–1400.
Bacigalupo A, Podesta M, Raffo MR, Piaggio G, Van Lint MT, Vimercati R et al. Lack of in vitro colony (CFUC) formation and myelosuppressive activity in patients with severe aplastic anemia after autologous hematologic reconstitution. Exp Hematol 1980; 8: 795–801.
Gupta V, Ball SE, Sage D, Ortin M, Freires M, Gordon-Smith EC et al. Marrow transplants from matched unrelated donors for aplastic anaemia using alemtuzumab, fludarabine and cyclophosphamide based conditioning. Bone Marrow Transplant 2005; 35: 467–471.
Gupta V, Ball SE, Yi QL, Sage D, McCann SR, Lawler M et al. Favorable effect on acute and chronic graft-versus-host disease with cyclophosphamide and in vivo anti-CD52 monoclonal antibodies for marrow transplantation from HLA-identical sibling donors for acquired aplastic anemia. Biol Blood Marrow Transplant 2004; 10: 867–876.
Siegal D, Xu W, Sutherland R, Kamel-Reid S, Kuruvilla J, Lipton JH et al. Graft-versus-host disease following marrow transplantation for aplastic anemia: different impact of two GVHD prevention strategies. Bone Marrow Transplant 2008; 42: 51–56.
Kennedy-Nasser AA, Leung KS, Mahajan A, Weiss HL, Arce JA, Gottschalk S et al. Comparable outcomes of matched-related and alternative donor stem cell transplantation for pediatric severe aplastic anemia. Biol Blood Marrow Transplant 2006; 12: 1277–1284.
Marsh JC, Ball SE, Darbyshire P, Gordon-Smith EC, Keidan AJ, Martin J et al. British Committee for Standards in Haematology (BCSH) General Haematology Task Force. Guidelines for the diagnosis and management of acquired aplastic anaemia. Br J Haematol 2003; 123: 782–801.
Selleri C, Maciejewski JP, Sato T, Young NS . Interferon gamma constitutively expressed in the stromal microenvironment of human marrow cultures mediates potent hematopoietic inhibition. Blood 1996; 87: 4149–4157.
Bacigalupo A, Valle M, Podesta M, Pitto A, Zocchi E, De Flora A et al. T-cell suppression mediated by mesenchymal stem cells is deficient in patients with severe aplastic anemia. Exp Hematol 2005; 33: 819–827.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Islam, M., Anoop, P., Datta-Nemdharry, P. et al. Implications of CD34+ cell dose on clinical and haematological outcome of allo-SCT for acquired aplastic anaemia. Bone Marrow Transplant 45, 886–894 (2010). https://doi.org/10.1038/bmt.2009.267
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2009.267
Keywords
This article is cited by
-
Excellent outcome with a high proportion of mixed chimerism in patients with severe aplastic anemia treated with partially T-cell-depleted peripheral hematopoietic stem cell transplants
Bone Marrow Transplantation (2016)
-
Allogeneic stem cell transplantation using alemtuzumab-containing regimens in severe aplastic anemia
International Journal of Hematology (2013)