Abstract
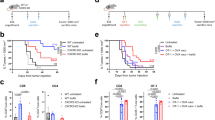
We have developed a vaccine, which is designed to induce tumor-associated antigen (TAA)-specific T cells and antibodies in the setting of profound lymphopenia induced by myeloablative therapy and T-cell-depleted bone marrow transplantation. Test mice were injected subcutaneously (sc) with the 32DP210Bcr-Abl cell line, which is positive for the p210Bcr-Abl protein (Group 1). In Group 2, 7 days after injection of the 32DP210Bcr-Abl positive cell line, the mice received 900 cGy total body irradiation (TBI) followed in 1 h by the intravenous infusion of 10 million T-cell-depleted syngeneic bone marrow cells (TCDBMT) (Group 2). The leukemia-bearing group received an intravenous injection of 10 million spleen cells (donor lymphocyte infusions) from unvaccinated (Group 3) and TAA/ecdCD40L-vaccinated (Group 4) syngeneic mice 3 days after completion of the TBI and TCDBMT. Groups 3 and 4 mice received three additional sc vaccinations at 7-day intervals with the TAA/ecdCD40L vaccine, in which the TAA was taken from the junctional peptide of the P210bcr-Abl protein. The survival of Groups 3 and 4 mice was significantly longer than that in Groups 1 and 2 mice. Vaccinated mice from Group 4, which developed complete responses, survived up to 350 days post-injection of the leukemia cells without any evidence of leukemia regrowth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jefferson T, Rivetti A, Rudin M, Di Pietrantonj D, Demicheli V . Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 2005; 366: 1165–1174.
Deng Y, Jing Y, Campbell AE, Gravenstein S . Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol 2004; 172: 3437–3446.
Castle SC . Clinical relevance of age-related immune dysfunction. Clin Infect Dis 2000; 31: 578–581.
Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I et al. Lack of antibody production following immunization in old age: association with CD8+CD28− T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol 2000; 168: 5893–5899.
Zhang L, Tang Y, Akbulut H, Zelterman D, Linton PJ, Deisseroth A . An adenoviral vector cancer vaccine that delivers a tumor-associated antigen/CD40-ligand fusion protein to dendritic cells. Proc Natl Acad Sci USA 2003; 100: 15101–15106.
Tang Y, Zhang L, Yuan J, Akbulut H, Linton PJ, Deisseroth A . Multistep process through which adenoviral vector vaccine overcomes anergy to tumor-associated antigens. Blood 2004; 104: 2704–2713.
Tang Y, Akbulut H, Koziol J, Linton PJ, Deisseroth A . Vector prime/protein boost vaccine that overcomes defects acquired during aging and cancer. J Immunol 2006; 177: 5697–5707.
Akbulut H, Tang YC, Akbulut KG, Maynard J, Zhang L, Deisseroth A . Antitumor immune response induced by i.t. injection of vector activated dendritic cells and chemotherapy suppresses metastatic breast cancer. Mol Cancer Ther 2006; 5: 1975–1985.
Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L . Age-related defects in CD4 T cells cognate helper function lead to reductions in humoral responses. J Exp Med 2004; 200: 1613–1622.
Dong L, Mori I, Hossain J, Liu B, Kimura Y . An immunostimulatory oligodeoxynucleotide containing a cytidine-guanosine motif protects senescence-accelerated mice from lethal influenza virus by augmenting the T helper type 1 response. J Gen Virol 2003; 84: 1623–1628.
Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst 1995; 87: 796–802.
Rhodes J, York RD, Tara D, Tajinda K, Druker BJ . CrkL functions as a nuclear adaptor and transcriptional activator in Bcr-Abl-expressing cells. Exp Hematol 2000; 28: 305–310.
La Rosche P, Johnson K, O’Dwyer ME, Druker BJ . In vitro studies of the combination of imatinib mesylate (Gleevec) and arsenic trioxide (Trisenox) in chronic myelogenous leukemia. Exp Hematol 2002; 30: 729–737.
Yasukawa M, Ohminami H, Kojima K, Hato T, Hasegawa A, Takahashi T et al. HLA class II-restricted antigen presentation of endogenous bcr-abl fusion protein by chronic myelogenous leukemia-derived dendritic cells to CD4+ T lymphocytes. Blood 2001; 98: 1498–1505.
Pinilla-Ibarz J, Cathcart K, Korontsvit T, Soignet S, Bocchia M, Caggiano J et al. Vaccination of patients with chronic myelogenous leukemia with bcr-abl oncogene breakpoint fusion peptides generates specific immune responses. Blood 2000; 95: 1781–1787.
Sun JY, Krouse RS, Forman SJ, Senitzer D, Sniecinski I, Chatterjee S et al. Immunogenecity of a p210BCR-ABL fusion domain candidate DNA vaccine targeted to dendritic cells by a recombinant adeno-associated virus vector in vitro. Cancer Res 2002; 62: 3175–3183.
Zeng Y, Graner MW, Thompson S, Marron M, Katsanis E . Induction of BCR-ABL-specific immunity following vaccination with chaperon-rich cell lysates derived from BCR-ABL+ tumor cells. Blood 2005; 105: 2016–2022.
Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME . Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008; 8: 299–308.
Acknowledgements
The SKCC and AD thank the Kaye and Richard Woltman Foundation for support of the development of post-allograft vaccination methods designed to reduce the post-allograft incidence of recurrence and opportunistic viral infections. AD recognizes support of the vaccine program from the Breast Cancer Research Foundation, the DOD (BCRP program (BC022063)), and a grant from the State of California Breast Cancer Research Program (CBCRP12IB-0159). Authors thank Dr Brian Druker for 32Dp210Bcrabl cells. Dr Tae Hae Han recognizes support from SBRI grant B-A7-204 and SRC grant from Korea Science and Engineering Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, T., Tang, Y., Park, Y. et al. Vector prime protein boost vaccination in the setting of myeloablative-induced lymphopenia suppresses growth of leukemia and solid tumors. Bone Marrow Transplant 45, 550–557 (2010). https://doi.org/10.1038/bmt.2009.185
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2009.185
Keywords
This article is cited by
-
TAA/ecdCD40L adenoviral prime-protein boost vaccine for cancer and infectious diseases
Cancer Gene Therapy (2013)