Abstract
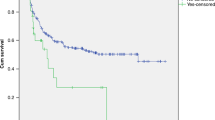
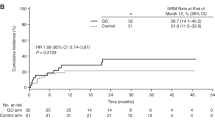
This was a retrospective multicenter study including 44 acute leukaemia patients who have received allogeneic haematopoietic SCT (allo-HSCT) after prior exposure to Gemtuzumab Ozogamicin (GO) + chemotherapy. Median interval between last administration of GO and allo-HSCT was 4.2 (range, 0.8–26.3) months. At time of allo-HSCT, 33 patients were in CR. The majority of patients (n=36) received a reduced-intensity conditioning (RIC) regimen before allo-HSCT. All but one patient received low-dose heparin for veno-occlusive disease (VOD) prophylaxis. With a median follow-up of 15 (range, 1.1–63) months, overall survival and disease-free survival after allo-HSCT were 45% (95% confidence interval (CI), 30–61%) and 38% (95% CI, 24–54%) at 2 years, respectively. The cumulative incidence of grade 3–4 hyperbilirubinemia was 13.5% (n=6), with this being 21% in patients with a short (⩽3.5 months) GO-allo-HSCT interval (n=4/19) vs 8% in all others (P=NS). Overall, the cumulative incidence of VOD was 7% (n=3), with this being 10.5% (n=2/19) in patients with a short GO-allograft interval (⩽3.5 months) vs 4% (n=1/25) for all others (P=NS), and 5.5% (n=2/36) in patients receiving an RIC regimen vs 12.5% for the others (n=1/8) (P=NS). These results suggest that GO-based chemotherapy before allo-HSCT is feasible and does not result in an excessive rate of liver toxicity, especially VOD, after allo-HSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Burnett AK, Kell WJ, Goldstone AH, Milligan D, Hunter A, Prentice AG et al. The addition of Gemtuzumab Ozogamicin to induction chemotherapy for AML improves disease free survival without extra toxicity: Preliminary analysis of 1115 patients in the MRC AML15 trial. Blood 2006; 108: 8a, abstract 13.
Chevallier P, Delaunay J, Turlure P, Pigneux A, Hunault M, Garand R et al. Long-term disease-free survival after Gemtuzumab, intermediate-dose cytarabine and Mitoxantrone in patients with CD33+ primary resistant or relapsed acute myeloid leukaemia. J Clin Oncol 2008; 26: 5192–5197.
Senzolo M, Germani G, Cholongitas E, Burra P, Burroughs AK . Veno occlusive disease: update on clinical management. World J Gastroenterol 2007; 13: 3918–3924.
Larson RA, Sievers EL, Stadtmauer EA, Löwenberg B, Estey EH, Dombret H et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer 2005; 104: 1442–1452.
Chevallier P, Roland V, Mahe B, Juge-Morineau N, Dubruille V, Guillaume T et al. Administration of mylotarg 4 days after beginning of a chemotherapy including intermediate-dose aracytin and mitoxantrone (MIDAM regimen) produces a high rate of complete hematologic remission in patients with CD33+ primary resistant or relapsed acute myeloid leukaemia. Leuk Res 2005; 29: 1003–1007.
Wadleigh M, Richardson PG, Zahrieh D, Lee SJ, Cutler C, Ho V et al. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood 2003; 102: 1578–1582.
Arceci RJ, Sande J, Lange B, Shannon K, Franklin J, Hutchinson R et al. Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33+acute myeloid aeukemia. Blood 2005; 106: 1183–1188.
McDonald G, Hinds M, Fisher L, Schoch HG, Wolford JL, Banaji M et al. Venoocclusive disease of the liver and multiorgan failure after bone marrow transplantation: A cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood 1998; 92: 2322–2333.
Carreras E, Bertz H, Arcese W, Vernant JP, Tomas JF, Hagglund JF et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: A prospective cohort study of the European group for blood and marrow transplantation. European Group for blood and marrow transplantation chronic leukemia working party. Blood 1998; 92: 3599–3604.
Scott BL, Sandmaier BM . Reduced-intensity allogeneic bone marrow transplantation: outcomes with myeloid malignancies. Hematology, (Educational Program, ASH 2006) 2006; 2006: 381–389.
Cortes J, Tsimberidou AM, Alvarez R, Thomas D, Beran M, Kantarjian H et al. Mylotarg combined with topotecan and cytarabine in patients with refractory acute myelogenous leukaemia. Cancer Chemother Pharmacol 2002; 50: 497–500.
Taksin AL, Legrand O, Raffoux E, de Revel T, Thomas X, Contentin N et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukaemia: a prospective study of the alfa group. Leukemia 2006; 21: 66–71.
Eom KS, Kim HJ, Min WS, Lee S, Min CK, Cho BS et al. Gemtuzumab ozogamicin in combination with attenuated doses of standard induction chemotherapy can successfully induce complete remission without increasing toxicity in patients with acute myeloid leukaemia aged 55 or older. Eur J Haematol 2007; 79: 398–404.
Candoni A, Martinelli G, Toffoletti E, Chiarvesio A, Tiribelli M, Malagola M et al. Gemtuzumab-ozogamicin in combination with fludarabine, cytarabine, idarubicin (FLAI-GO) as induction therapy in CD33-positive AML patients younger than 65 years. Leuk Res 2008; 32: 1800–1808.
Aplenc R, Alonzo TA, Gerbing RB, Lange BJ, Hurwitz CA, Wells RJ et al. Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukaemia: a report from the children 's oncology group. J Clin Oncol 2008; 26: 2390–3295.
Brethon B, Yakouben K, Oudot C, Boutard P, Bruno B, Jerome C et al. Efficacy of fractionated gemtuzumab ozogamicin combined with cytarabine in advanced childhood myeloid leukaemia. Br J Haematol 2008; 143: 541–547.
De Lima M, Champlin RE, Thall PF, Wang X, Martin 3rd TG, Cook JD et al. Phase I/II study of gemtuzumab ozogamicin added to fludarabine, melphalan and allogeneic hematopoietic stem cell transplantation for high-risk CD33 positive myeloid leukemias and myelodysplastic syndrome. Leukemia 2008; 22: 258–264.
Bornhauser M, Illmer T, Oelschlaegel U, Schetelig J, Ordemann R, Schaich M et al. Gemtuzumab ozogamicin as part of reduced-intensity conditioning for allogeneic hematopoietic cell transplantation in patients with relapsed acute myeloid leukemia. Clin Cancer Res 2008; 14: 5585–5593.
Attal M, Huguet F, Rubie H, Huynh A, Charlet JP, Payen JL et al. Prevention of hepatic veno-occlusive disease after bone marrow transplantation by continuous infusion of low-dose heparin: a prospective, randomized trial. Blood 1992; 79: 2834–2840.
Cesaro S, Pillon M, Talenti E, Toffolutti T, Calore E, Biasolo MA et al. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Haematologica 2005; 90: 1396–1404.
Versluys B, Bhattacharaya R, Steward C, Cornish J, Oakhill A, Goulden N . Prophylaxis with defibrotide prevents veno-occlusive disease in stem cell transplantation after gemtuzumab ozogamicin exposure. Blood 2004; 103: 1968.
Mohty M, Bay JO, Faucher C, Choufi B, Bilger K, Tournilhac O et al. Graft-versus-host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin-based reduced-intensity preparative regimen. Blood 2003; 102: 470–476.
Van der Velden VH, Boeckx N, Jedema I, te Marvelde JG, Hoogeveen PG, Boogaerts M et al. High CD33-antigen loads in peripheral blood limit the efficacy of gemtuzumab ozogamicin (Mylotarg) treatment in acute myeloid leukemia patients. Leukemia 2004; 18: 983–988.
McDonald GB . Management of hepatic sinusoidal obstruction syndrome following treatment with gemtuzumab ozogamicin (Mylotarg). Clin Lymphoma 2002; 2: S35–S39.
Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D et al. Long-term survival in refractory acute myeloid leukaemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 2006; 108: 1092–1099.
Acknowledgements
We thank the nursing staff for providing excellent care for our patients. We also thank the ‘Région Pays de Loire’, the ‘Association pour la Recherche sur le Cancer (ARC)’, the ‘Fondation de France’, the ‘Fondation contre la Leucémie’, the ‘Agence de Biomédecine’, the ‘Association Cent pour Sang la Vie’ and the ‘Association Laurette Fuguain’ for their generous and continuous support for our clinical and basic research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chevallier, P., Prebet, T., Turlure, P. et al. Prior treatment with gemtuzumab ozogamicin and the risk of veno-occlusive disease after allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant 45, 165–170 (2010). https://doi.org/10.1038/bmt.2009.153
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2009.153
Keywords
This article is cited by
-
Risk of sinusoidal obstruction syndrome in allogeneic stem cell transplantation after prior gemtuzumab ozogamicin treatment: a retrospective study from the Acute Leukemia Working Party of the EBMT
Bone Marrow Transplantation (2017)
-
Recent Advances in the Development of Antineoplastic Agents Derived from Natural Products
Drugs (2015)
-
Recent decrease in non-relapse mortality due to GVHD and infection after allogeneic hematopoietic cell transplantation in non-remission acute leukemia
Bone Marrow Transplantation (2013)
-
Fractionated doses of gemtuzumab ozogamicin combined with 3 + 7 induction chemotherapy as salvage treatment for young patients with acute myeloid leukemia in first relapse
Annals of Hematology (2012)
-
Therapeutic Sesamol Attenuates Monocrotaline-Induced Sinusoidal Obstruction Syndrome in Rats by Inhibiting Matrix Metalloproteinase-9
Cell Biochemistry and Biophysics (2011)