Abstract
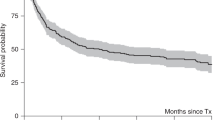
We found earlier that high-dose chemotherapy with Allo-SCT produced a tumor response in patients with chemorefractory metastatic breast cancer. In this study, we examined the efficacy and toxicity of nonmyeloablative allogeneic PBSC transplantation in patients with chemosensitive metastatic breast cancer. Twelve patients with metastatic breast carcinoma who had stable disease after standard-dose chemotherapy and six who had a partial response underwent allogeneic transplantation. The conditioning regimen consisted of reduced-intensity fludarabine and melphalan. All patients achieved engraftment and hematopoietic recovery. Nine patients developed grade II or higher acute GVHD; seven of these nine responded to immunosuppressive therapy. Fourteen patients developed chronic GVHD. The treatment-related mortality rate was 11%. With a median follow-up of 565 days, the median survival duration was 643 days and the median progression-free survival duration was 202 days. Two patients are alive with a complete response 1555 and 2526 days after SCT, and one patient is alive with progressive bone disease at day 1118. We conclude that among patients with chemotherapy-sensitive metastatic breast cancer, a fraction will achieve a durable complete response after SCT with a reduced-intensity conditioning regimen. The question remains how to improve the overall efficacy and reduce the mortality rate for this approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hortobagyi GN . Can we cure limited metastatic breast cancer? J Clin Oncol 2002; 20: 620–623.
Greenberg PA, Hortobagyi GN, Smith TL, Ziegler LD, Frye DK, Buzdar AU . Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol 1996; 14: 2197–2205.
Ben-Yosef R, Or R, Nagler A, Slavin S . Graft-versus-tumour and graft-versus-leukaemia effect in patient with concurrent breast cancer and acute myelocytic leukaemia. Lancet 1996; 348: 1242–1243.
Eibl B, Schwaighofer H, Nachbaur D, Marth C, Gachter A, Knapp R et al. Evidence for a graft-versus-tumor effect in a patient treated with marrow ablative chemotherapy and allogeneic bone marrow transplantation for breast cancer. Blood 1996; 88: 1501–1508.
Ueno NT, Rondon G, Mirza NQ, Geisler DK, Anderlini P, Giralt SA et al. Allogeneic peripheral-blood progenitor-cell transplantation for poor-risk patients with metastatic breast cancer. J Clin Oncol 1998; 16: 986–993.
Moscovitch M, Slavin S . Anti-tumor effects of allogeneic bone marrow transplantation in (NZB × NZW)F1 hybrids with spontaneous lymphosarcoma. J Immunol 1984; 132: 997–1000.
Prigozhina TB, Gurevitch O, Morecki S, Yakovlev E, Elkin G, Slavin S . Nonmyeloablative allogeneic bone marrow transplantation as immunotherapy for hematologic malignancies and metastatic solid tumors in preclinical models. Exp Hematol 2002; 30: 89–96.
Blaise D, Bay JO, Faucher C, Michallet M, Boiron J-M, Choufi B et al. Reduced-intensity preparative regimen and allogeneic stem cell transplantation for advanced solid tumors. Blood 2004; 103: 435–441.
Bregni M, Dodero A, Peccatori J, Pescarollo A, Bernardi M, Sassi I . Nonmyeloablative conditioning followed by hematopoietic cell allografting and donor lymphocyte infusions for patients with metastatic renal and breast cancer. Blood 2002; 99: 4234–4236.
Carella AM, Beltrami G, Lerma E, Cavaliere M, Corsetti MT . Combined use of autografting and non-myeloablative allografting for the treatment of hematologic malignancies and metastatic breast cancer. Cancer Treat Res 2002; 110: 101–112.
Ueno NT, Cheng YC, Rondon G, Tannir NM, Gajewski JL, Couriel DR et al. Rapid induction of complete donor chimerism by the use of a reduced-intensity conditioning regimen composed of fludarabine and melphalan in allogeneic stem cell transplantation for metastatic solid tumors. Blood 2003; 102: 3829–3836.
Schwartz DW, Glock B, Jungl EM, Mayr WR . Strategy to detect chimerism in allogeneic bone marrow transplant recipients by PCR-amplification fragment length polymorphism analysis of microsatellite polymorphisms. Vox Sang 1995; 68: 139–143.
Yam PY, Petz LD, Knowlton RG, Wallace RB, Stock AD, de Lange G et al. Use of DNA restriction fragment length polymorphisms to document marrow engraftment and mixed hematopoietic chimerism following bone marrow transplantation. Transplantation 1987; 43: 399–407.
An International System for Human Cytogenetic Nomenclature (1985) ISCN 1985. Report of the Standing Committee on Human Cytogenetic Nomenclature. Birth Defects: Orig Artic Ser 1985; 21: 1–117.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Imanguli MM, Childs RW . Hematopoietic stem cell transplantation for solid tumors. Update Cancer Ther 2006; 1: 343–352.
Oblon DJ, Paul S, Yankee R . Allogeneic transplantation after a conditioning regimen with ifosfamide, carboplatin and etoposide (ICE). Bone Marrow Transplant 1997; 20: 421–423.
Carella AM, Beltrami G, Corsetti MT, Nati S, Musto P, Scalzulli P et al. Reduced intensity conditioning for allograft after cytoreductive autograft in metastatic breast cancer. Lancet 2005; 366: 318–320.
Carella AM, Bregni M . Current role of allogeneic stem cell transplantation in breast cancer. Ann Oncol 2007; 18: 1591–1593.
Carella AM, Ferrara R, Orcioni GF, Pepe G, Villavecchia G . Profound graft-versus-tumor response in metastatic breast cancer with nonmyeloablative allografting. Ann Oncol 2007; 18: 1751–1754.
Bishop MR, Fowler DH, Marchigiani D, Castro K, Kasten-Sportes C, Steinberg SM et al. Allogeneic lymphocytes induce tumor regression of advanced metastatic breast cancer. J Clin Oncol 2004; 22: 3886–3892.
Ueno NT, Rizzo JD, Demirer T, Cheng YC, Hegenbart U, Zhang MJ et al. Allogeneic hematopoietic cell transplantation for metastatic breast cancer. Bone Marrow Transplant 2008; 41: 537–545.
Acknowledgements
We thank Sunita Patterson of the Department of Scientific Publications at M.D. Anderson Cancer Center for her expert editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Souza, J., Davis, M., Rondon, G. et al. Prolonged disease control by nonmyeloablative allogeneic transplantation for metastatic breast cancer. Bone Marrow Transplant 44, 81–87 (2009). https://doi.org/10.1038/bmt.2009.101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2009.101
Keywords
This article is cited by
-
Carcinomatosis lymphangitis and pleurisy after allo-SCT in two patients with secondary leukemia after breast cancer
Bone Marrow Transplantation (2012)