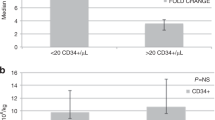
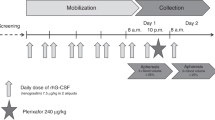
Abstract
Autologous hematopoietic SCT (auto-HSCT) provides hematopoietic support after high-dose chemotherapy and is the standard of care for patients with multiple myeloma (MM) or chemosensitive relapsed high- or intermediate-grade non-Hodgkin's lymphoma (NHL). However, yields of hematopoietic stem cells vary greatly between patients, and the optimal strategy to mobilize hematopoietic stem cells into peripheral blood for collection has not been defined. Current mobilization strategies consist of cytokines alone or in combination with chemotherapeutic agents. Cytokine-only mobilization regimens are well tolerated, but their utility is limited by suboptimal PBSC yields. When a myelosuppressive chemotherapeutic agent is added to a cytokine mobilization regimen, PBSC collections improve two- to five-fold. This benefit is tempered by increased toxicity, morbidity and resource utilization. All current regimens fail to mobilize sufficient hematopoietic stem cells to proceed to transplantation in 5–30% of patients, necessitating additional mobilization attempts or precluding transplantation, which may negatively affect patient outcomes and survival. Improved strategies to mobilize stem cells would increase the availability of auto-HSCT and optimize engraftment and outcomes in patients with MM or NHL. Novel agents used in conjunction with cytokines have the potential to increase PBSC collections without introducing additional morbidity, thereby improving patient outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hennessy BT, Hanrahan EO, Daly PA . Non-Hodgkin lymphoma: an update. Lancet Oncol 2004; 5: 341–353.
Villanueva ML, Vose JM . The role of hematopoietic stem cell transplantation in non-Hodgkin lymphoma. Clin Adv Hematol Oncol 2006; 4: 521–530.
Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med 1995; 333: 1540–1545.
Harousseau JL, Moreau P . Role of bone marrow transplantation in the disease pathway of myeloma. J Natl Compr Canc Netw 2007; 5: 163–169.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–1883.
Hari P, Pasquini MC, Vesole DH . Cure of multiple myeloma—more hype, less reality. Bone Marrow Transplant 2006; 37: 1–18.
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335: 91–97.
O'Shea D, Giles C, Terpos E, Perz J, Politou M, Sana V et al. Predictive factors for survival in myeloma patients who undergo autologous stem cell transplantation: a single-centre experience in 211 patients. Bone Marrow Transplant 2006; 37: 731–737.
Dingli D, Pacheco JM, Dispenzieri A, Hayman SR, Kumar SK, Lacy MQ et al. Serum M-spike and transplant outcome in patients with multiple myeloma. Cancer Sci 2007; 98: 1035–1040.
Rohatiner AZ, Nadler L, Davies AJ, Apostolidis J, Neuberg D, Matthews J et al. Myeloablative therapy with autologous bone marrow transplantation for follicular lymphoma at the time of second or subsequent remission: long-term follow-up. J Clin Oncol 2007; 25: 2554–2559.
Jillella AP, Ustun C . What is the optimum number of CD34+ peripheral blood stem cells for an autologous transplant? Stem Cells Dev 2004; 13: 598–606.
Siena S, Schiavo R, Pedrazzoli P, Carlo-Stella C . Therapeutic relevance of CD34 cell dose in blood cell transplantation for cancer therapy. J Clin Oncol 2000; 18: 1360–1377.
Tricot G, Jagannath S, Vesole D, Nelson J, Tindle S, Miller L et al. Peripheral blood stem cell transplants for multiple myeloma: identification of favorable variables for rapid engraftment in 225 patients. Blood 1995; 85: 588–596.
Stiff PJ . Management strategies for the hard-to-mobilize patient. Bone Marrow Transplant 1999; 23 (Suppl 2): S29–S33.
Sola C, Maroto P, Salazar R, Mesia R, Mendoza L, Brunet J et al. Bone marrow transplantation: prognostic factors of peripheral blood stem cell mobilization with cyclophosphamide and filgrastim (r-metHuG-CSF): the CD34+ cell dose positively affects the time to hematopoietic recovery and supportive requirements after high-dose chemotherapy. Hematology 1999; 4: 195–209.
Duggan PR, Guo D, Luider J, Auer I, Klassen J, Chaudhry A et al. Predictive factors for long-term engraftment of autologous blood stem cells. Bone Marrow Transplant 2000; 26: 1299–1304.
Gordan LN, Sugrue MW, Lynch JW, Williams KD, Khan SA, Wingard JR et al. Poor mobilization of peripheral blood stem cells is a risk factor for worse outcome in lymphoma patients undergoing autologous stem cell transplantation. Leuk Lymphoma 2003; 44: 815–820.
Toor AA, Ayers J, Strupeck J, Parthasarathy M, Creech S, Rodriguez T et al. Favourable results with a single autologous stem cell transplant following conditioning with busulphan and cyclophosphamide in patients with multiple myeloma. Br J Haematol 2004; 124: 769–776.
Ikeda K, Kozuka T, Harada M . Factors for PBPC collection efficiency and collection predictors. Transfus Apher Sci 2004; 31: 245–259.
Olavarria E, Kanfer EJ . Selection and use of chemotherapy with hematopoietic growth factors for mobilization of peripheral blood progenitor cells. Curr Opin Hematol 2000; 7: 191–196.
Nervi B, Link DC, DiPersio JF . Cytokines and hematopoietic stem cell mobilization. J Cell Biochem 2006; 99: 690–705.
Sugrue MW, Williams K, Pollock BH, Khan S, Peracha S, Wingard JR et al. Characterization and outcome of ‘hard to mobilize’ lymphoma patients undergoing autologous stem cell transplantation. Leuk Lymphoma 2000; 39: 509–519.
Boeve S, Strupeck J, Creech S, Stiff PJ . Analysis of remobilization success in patients undergoing autologous stem cell transplants who fail an initial mobilization: risk factors, cytokine use and cost. Bone Marrow Transplant 2004; 33: 997–1003.
Goterris R, Hernandez-Boluda JC, Teruel A, Gomez C, Lis MJ, Terol MJ et al. Impact of different strategies of second-line stem cell harvest on the outcome of autologous transplantation in poor peripheral blood stem cell mobilizers. Bone Marrow Transplant 2005; 36: 847–853.
Bensinger W, Appelbaum F, Rowley S, Storb R, Sanders J, Lilleby K et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol 1995; 13: 2547–2555.
Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood 1995; 86: 3961–3969.
Alegre A, Tomas JF, Martinez-Chamorro C, Gil-Fernandez JJ, Fernandez-Villalta MJ, Arranz R et al. Comparison of peripheral blood progenitor cell mobilization in patients with multiple myeloma: high-dose cyclophosphamide plus GM-CSF vs G-CSF alone. Bone Marrow Transplant 1997; 20: 211–217.
Narayanasami U, Kanteti R, Morelli J, Klekar A, Al-Olama A, Keating C et al. Randomized trial of filgrastim versus chemotherapy and filgrastim mobilization of hematopoietic progenitor cells for rescue in autologous transplantation. Blood 2001; 98: 2059–2064.
Desikan KR, Barlogie B, Jagannath S, Vesole DH, Siegel D, Fassas A et al. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. J Clin Oncol 1998; 16: 1547–1553.
Tarella C, Zallio F, Caracciolo D, Cherasco C, Bondesan P, Gavarotti P et al. Hematopoietic progenitor cell mobilization and harvest following an intensive chemotherapy debulking in indolent lymphoma patients. Stem Cells 1999; 17: 55–61.
Winkler IG, Levesque JP . Mechanisms of hematopoietic stem cell mobilization: when innate immunity assails the cells that make blood and bone. Exp Hematol 2006; 34: 996–1009.
King AG, Horowitz D, Dillon SB, Levin R, Farese AM, MacVittie TJ et al. Rapid mobilization of murine hematopoietic stem cells with enhanced engraftment properties and evaluation of hematopoietic progenitor cell mobilization in rhesus monkeys by a single injection of SB-251353, a specific truncated form of the human CXC chemokine GRObeta. Blood 2001; 97: 1534–1542.
Pelus LM, Fukuda S . Peripheral blood stem cell mobilization: the CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties. Exp Hematol 2006; 34: 1010–1020.
Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A et al. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet 1996; 347: 353–357.
Gazitt Y . Comparison between granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in the mobilization of peripheral blood stem cells. Curr Opin Hematol 2002; 9: 190–198.
Haas R, Mohle R, Fruhauf S, Goldschmidt H, Witt B, Flentje M et al. Patient characteristics associated with successful mobilizing and autografting of peripheral blood progenitor cells in malignant lymphoma. Blood 1994; 83: 3787–3794.
Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzow MR et al. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma 2006; 6: 384–388.
Ford CD, Greenwood J, Anderson J, Snow G, Petersen FB . CD34+ cell adhesion molecule profiles differ between patients mobilized with granulocyte-colony-stimulating factor alone and chemotherapy followed by granulocyte-colony-stimulating factor. Transfusion 2006; 46: 193–198.
Koc ON, Gerson SL, Cooper BW, Laughlin M, Meyerson H, Kutteh L et al. Randomized cross-over trial of progenitor-cell mobilization: high-dose cyclophosphamide plus granulocyte colony-stimulating factor (G-CSF) versus granulocyte-macrophage colony-stimulating factor plus G-CSF. J Clin Oncol 2000; 18: 1824–1830.
Fitoussi O, Perreau V, Boiron JM, Bouzigon E, Cony-Makhoul P, Pigneux A et al. A comparison of toxicity following two different doses of cyclophosphamide for mobilization of peripheral blood progenitor cells in 116 multiple myeloma patients. Bone Marrow Transplant 2001; 27: 837–842.
Jantunen E, Putkonen M, Nousiainen T, Pelliniemi TT, Mahlamaki E, Remes K . Low-dose or intermediate-dose cyclophosphamide plus granulocyte colony-stimulating factor for progenitor cell mobilisation in patients with multiple myeloma. Bone Marrow Transplant 2003; 31: 347–351.
Gupta S, Zhou P, Hassoun H, Kewalramani T, Reich L, Costello S et al. Hematopoietic stem cell mobilization with intravenous melphalan and G-CSF in patients with chemoresponsive multiple myeloma: report of a phase II trial. Bone Marrow Transplant 2005; 35: 441–447.
Hicks ML, Lonial S, Langston A, Flowers C, Roback JD, Smith KJ et al. Optimizing the timing of chemotherapy for mobilizing autologous blood hematopoietic progenitor cells. Transfusion 2007; 47: 629–635.
Bargetzi MJ, Passweg J, Baertschi E, Schoenenberger A, Gwerder C, Tichelli A et al. Mobilization of peripheral blood progenitor cells with vinorelbine and granulocyte colony-stimulating factor in multiple myeloma patients is reliable and cost effective. Bone Marrow Transplant 2003; 31: 99–103.
Tarella C, Di Nicola M, Caracciolo D, Zallio F, Cuttica A, Omede P et al. High-dose ara-C with autologous peripheral blood progenitor cell support induces a marked progenitor cell mobilization: an indication for patients at risk for low mobilization. Bone Marrow Transplant 2002; 30: 725–732.
Dreger P, Kloss M, Petersen B, Haferlach T, Loffler H, Loeffler M et al. Autologous progenitor cell transplantation: prior exposure to stem cell-toxic drugs determines yield and engraftment of peripheral blood progenitor cell but not of bone marrow grafts. Blood 1995; 86: 3970–3978.
Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant 2008; 14: 1045–1056.
Cashen AF, Link D, Devine S, DiPersio J . Cytokines and stem cell mobilization for autologous and allogeneic transplantation. Curr Hematol Rep 2004; 3: 406–412.
Nakamura Y, Tajima F, Ishiga K, Yamazaki H, Oshimura M, Shiota G et al. Soluble c-kit receptor mobilizes hematopoietic stem cells to peripheral blood in mice. Exp Hematol 2004; 32: 390–396.
Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM, Harlan JM . Synergistic mobilization of hematopoietic progenitor cells using concurrent beta1 and beta2 integrin blockade or beta2-deficient mice. Blood 2001; 97: 1282–1288.
Craddock CF, Nakamoto B, Andrews RG, Priestley GV, Papayannopoulou T . Antibodies to VLA4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. Blood 1997; 90: 4779–4788.
Pecora AL . Impact of stem cell dose on hematopoietic recovery in autologous blood stem cell recipients. Bone Marrow Transplant 1999; 23 (Suppl 2): S7–S12.
Opie TM, Shields LE, Andrews RG . Cell-surface antigen expression in early and term gestation fetal hematopoietic progenitor cells. Stem Cells 1998; 16: 343–348.
Dao MA, Arevalo J, Nolta JA . Reversibility of CD34 expression on human hematopoietic stem cells that retain the capacity for secondary reconstitution. Blood 2003; 101: 112–118.
Fu S, Liesveld J . Mobilization of hematopoietic stem cells. Blood Rev 2000; 14: 205–218.
Hartmann O, Le Corroller AG, Blaise D, Michon J, Philip I, Norol F et al. Peripheral blood stem cell and bone marrow transplantation for solid tumors and lymphomas: hematologic recovery and costs. A randomized, controlled trial. Ann Intern Med 1997; 126: 600–607.
Beyer J, Schwella N, Zingsem J, Strohscheer I, Schwaner I, Oettle H et al. Hematopoietic rescue after high-dose chemotherapy using autologous peripheral-blood progenitor cells or bone marrow: a randomized comparison. J Clin Oncol 1995; 13: 1328–1335.
Gandhi MK, Jestice K, Scott MA, Bloxham D, Bass G, Marcus RE . The minimum CD34 threshold depends on prior chemotherapy in autologous peripheral blood stem cell recipients. Bone Marrow Transplant 1999; 23: 9–13.
Montgomery M, Cottler-Fox M . Mobilization and collection of autologous hematopoietic progenitor/stem cells. Clin Adv Hematol Oncol 2007; 5: 127–136.
Pavone V, Gaudio F, Console G, Vitolo U, Iacopino P, Guarini A et al. Poor mobilization is an independent prognostic factor in patients with malignant lymphomas treated by peripheral blood stem cell transplantation. Bone Marrow Transplant 2006; 37: 719–724.
Bolwell BJ, Pohlman B, Rybicki L, Sobecks R, Dean R, Curtis J et al. Patients mobilizing large numbers of CD34+ cells (‘super mobilizers’) have improved survival in autologous stem cell transplantation for lymphoid malignancies. Bone Marrow Transplant 2007; 40: 437–441.
Wahlin A, Eriksson M, Hultdin M . Relation between harvest success and outcome after autologous peripheral blood stem cell transplantation in multiple myeloma. Eur J Haematol 2004; 73: 263–268.
Dazzi C, Cariello A, Rosti G, Argnani M, Sebastiani L, Ferrari E et al. Is there any difference in PBPC mobilization between cyclophosphamide plus G-CSF and G-CSF alone in patients with non-Hodgkin's lymphoma? Leuk Lymphoma 2000; 39: 301–310.
Leukine (sargramostim) [package insert]. Berlex: Seattle, WA, 2006.
Neupogen (filgrastim) [package insert]. Amgen Inc.: Thousand Oaks, CA, 1991–2006.
Metcalf D . The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood 1986; 67: 257–267.
Welte K, Platzer E, Lu L, Gabrilove JL, Levi E, Mertelsmann R et al. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci USA 1985; 82: 1526–1530.
Gazitt Y, Shaughnessy P, Liu Q . Differential mobilization of CD34+ cells and lymphoma cells in non-Hodgkin's lymphoma patients mobilized with different growth factors. J Hematother Stem Cell Res 2001; 10: 167–176.
Socinski MA, Cannistra SA, Elias A, Antman KH, Schnipper L, Griffin JD . Granulocyte-macrophage colony stimulating factor expands the circulating haematopoietic progenitor cell compartment in man. Lancet 1988; 1: 1194–1198.
Arora M, Burns LJ, Barker JN, Miller JS, Defor TE, Olujohungbe AB et al. Randomized comparison of granulocyte colony-stimulating factor versus granulocyte-macrophage colony-stimulating factor plus intensive chemotherapy for peripheral blood stem cell mobilization and autologous transplantation in multiple myeloma. Biol Blood Marrow Transplant 2004; 10: 395–404.
Weaver CH, Schulman KA, Wilson-Relyea B, Birch R, West W, Buckner CD . Randomized trial of filgrastim, sargramostim, or sequential sargramostim and filgrastim after myelosuppressive chemotherapy for the harvesting of peripheral-blood stem cells. J Clin Oncol 2000; 18: 43–53.
Cottler-Fox MH, Lapidot T, Petit I, Kollet O, DiPersio JF, Link D et al. Stem cell mobilization. Hematology Am Soc Hematol Educ Program 2003; 419–437.
Verfaillie CM . Adhesion receptors as regulators of the hematopoietic process. Blood 1998; 92: 2609–2612.
Vermeulen M, Le Pesteur F, Gagnerault MC, Mary JY, Sainteny F, Lepault F . Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood 1998; 92: 894–900.
Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ . Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood 2001; 98: 1289–1297.
Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 2002; 109: 625–637.
Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C et al. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood 2004; 104: 65–72.
Levesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ . Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol 2002; 30: 440–449.
Pruijt JF, Verzaal P, van Os R, de Kruijf EJ, van Schie ML, Mantovani A et al. Neutrophils are indispensable for hematopoietic stem cell mobilization induced by interleukin-8 in mice. Proc Natl Acad Sci USA 2002; 99: 6228–6233.
Pelus LM, Bian H, King AG, Fukuda S . Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood 2004; 103: 110–119.
Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood 2005; 106: 3020–3027.
To LB, Haylock DN, Simmons PJ, Juttner CA . The biology and clinical uses of blood stem cells. Blood 1997; 89: 2233–2258.
Gazitt Y, Freytes CO, Callander N, Tsai TW, Alsina M, Anderson J et al. Successful PBSC mobilization with high-dose G-CSF for patients failing a first round of mobilization. J Hematother 1999; 8: 173–183.
Nademanee A, Sniecinski I, Schmidt GM, Dagis AC, O'Donnell MR, Snyder DS et al. High-dose therapy followed by autologous peripheral-blood stem-cell transplantation for patients with Hodgkin's disease and non-Hodgkin's lymphoma using unprimed and granulocyte colony-stimulating factor-mobilized peripheral-blood stem cells. J Clin Oncol 1994; 12: 2176–2186.
Pavone V, Gaudio F, Guarini A, Perrone T, Zonno A, Curci P et al. Mobilization of peripheral blood stem cells with high-dose cyclophosphamide or the DHAP regimen plus G-CSF in non-Hodgkin's lymphoma. Bone Marrow Transplant 2002; 29: 285–290.
Kanteti R, Miller K, McCann J, Roitman D, Morelli J, Hurley C et al. Randomized trial of peripheral blood progenitor cell vs bone marrow as hematopoietic support for high-dose chemotherapy in patients with non-Hodgkin's lymphoma and Hodgkin's disease: a clinical and molecular analysis. Bone Marrow Transplant 1999; 24: 473–481.
Schiller G, Vescio R, Freytes C, Spitzer G, Sahebi F, Lee M et al. Transplantation of CD34+ peripheral blood progenitor cells after high-dose chemotherapy for patients with advanced multiple myeloma. Blood 1995; 86: 390–397.
Moskowitz CH, Glassman JR, Wuest D, Maslak P, Reich L, Gucciardo A et al. Factors affecting mobilization of peripheral blood progenitor cells in patients with lymphoma. Clin Cancer Res 1998; 4: 311–316.
Micallef IN, Apostolidis J, Rohatiner AZ, Wiggins C, Crawley CR, Foran JM et al. Factors which predict unsuccessful mobilisation of peripheral blood progenitor cells following G-CSF alone in patients with non-Hodgkin's lymphoma. Hematol J 2000; 1: 367–373.
Kobbe G, Sohngen D, Bauser U, Schneider P, Germing U, Thiele KP et al. Factors influencing G-CSF-mediated mobilization of hematopoietic progenitor cells during steady-state hematopoiesis in patients with malignant lymphoma and multiple myeloma. Ann Hematol 1999; 78: 456–462.
Zeller W, Gutensohn K, Stockschlader M, Dierlamm J, Kroger N, Koehne G et al. Increase of mobilized CD34-positive peripheral blood progenitor cells in patients with Hodgkin's disease, non-Hodgkin's lymphoma, and cancer of the testis. Bone Marrow Transplant 1996; 17: 709–713.
Sheridan WP, Begley CG, To LB, Grigg A, Szer J, Maher D et al. Phase II study of autologous filgrastim (G-CSF)-mobilized peripheral blood progenitor cells to restore hemopoiesis after high-dose chemotherapy for lymphoid malignancies. Bone Marrow Transplant 1994; 14: 105–111.
Wang S, Nademanee A, Qian D, Dagis A, Park HS, Fridey J et al. Peripheral blood hematopoietic stem cell mobilization and collection efficacy is not an independent prognostic factor for autologous stem cell transplantation. Transfusion 2007; 47: 2207–2216.
Yasui K, Tsuno T, Miyabayashi M, Yamazaki M, Komiyama A . Effects of high-dose granulocyte colony-stimulating factor on neutrophil functions. Br J Haematol 1996; 92: 571–573.
Madero L, Vicent MG, Sevilla J, Prudencio M, Rodriguez F, Diaz MA . Engraftment syndrome in children undergoing autologous peripheral blood progenitor cell transplantation. Bone Marrow Transplant 2002; 30: 355–358.
Bot FJ, van Eijk L, Schipper P, Backx B, Lowenberg B . Synergistic effects between GM-CSF and G-CSF or M-CSF on highly enriched human marrow progenitor cells. Leukemia 1990; 4: 325–328.
Quittet P, Ceballos P, Lopez E, Lu ZY, Latry P, Becht C et al. Low doses of GM-CSF (molgramostim) and G-CSF (filgrastim) after cyclophosphamide (4 g/m2) enhance the peripheral blood progenitor cell harvest: results of two randomized studies including 120 patients. Bone Marrow Transplant 2006; 38: 275–284.
Gazitt Y, Callander N, Freytes CO, Shaughnessy P, Liu Q, Tsai TW et al. Peripheral blood stem cell mobilization with cyclophosphamide in combination with G-CSF, GM-CSF, or sequential GM-CSF/G-CSF in non-Hodgkin's lymphoma patients: a randomized prospective study. J Hematother Stem Cell Res 2000; 9: 737–748.
Bashey A, Corringham S, Gilpin E, Fields KK, Smilee RC, DeFrancisco C et al. Simultaneous administration of G-CSF and GM-CSF for re-mobilization in patients with inadequate initial progenitor cell collections for autologous transplantation. Cytotherapy 2000; 2: 195–200.
Spitzer G, Adkins D, Mathews M, Velasquez W, Bowers C, Dunphy F et al. Randomized comparison of G-CSF + GM-CSF vs G-CSF alone for mobilization of peripheral blood stem cells: effects on hematopoietic recovery after high-dose chemotherapy. Bone Marrow Transplant 1997; 20: 921–930.
Olivieri A, Offidani M, Cantori I, Ciniero L, Ombrosi L, Masia MC et al. Addition of erythropoietin to granulocyte colony-stimulating factor after priming chemotherapy enhances hematopoietic progenitor mobilization. Bone Marrow Transplant 1995; 16: 765–770.
Kaushansky K . Lineage-specific hematopoietic growth factors. N Engl J Med 2006; 354: 2034–2045.
Perillo A, Ferrandina G, Pierelli L, Rutella S, Mancuso S, Scambia G . Cytokines alone for PBPC collection in patients with advanced gynaecological malignancies: G-CSF vs G-CSF plus EPO. Bone Marrow Transplant 2004; 34: 743–744.
Miller CB, Lazarus HM . Erythropoietin in stem cell transplantation. Bone Marrow Transplant 2001; 27: 1011–1016.
Glaspy JA, Shpall EJ, LeMaistre CF, Briddell RA, Menchaca DM, Turner SA et al. Peripheral blood progenitor cell mobilization using stem cell factor in combination with filgrastim in breast cancer patients. Blood 1997; 90: 2939–2951.
Lapidot T, Petit I . Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol 2002; 30: 973–981.
Moskowitz CH, Stiff P, Gordon MS, McNiece I, Ho AD, Costa JJ et al. Recombinant methionyl human stem cell factor and filgrastim for peripheral blood progenitor cell mobilization and transplantation in non-Hodgkin's lymphoma patients—results of a phase I/II trial. Blood 1997; 89: 3136–3147.
Stiff P, Gingrich R, Luger S, Wyres MR, Brown RA, LeMaistre CF et al. A randomized phase 2 study of PBPC mobilization by stem cell factor and filgrastim in heavily pretreated patients with Hodgkin's disease or non-Hodgkin's lymphoma. Bone Marrow Transplant 2000; 26: 471–481.
To LB, Bashford J, Durrant S, MacMillan J, Schwarer AP, Prince HM et al. Successful mobilization of peripheral blood stem cells after addition of ancestim (stem cell factor) in patients who had failed a prior mobilization with filgrastim (granulocyte colony-stimulating factor) alone or with chemotherapy plus filgrastim. Bone Marrow Transplant 2003; 31: 371–378.
Dawson MA, Schwarer AP, Muirhead JL, Bailey MJ, Bollard GM, Spencer A . Successful mobilization of peripheral blood stem cells using recombinant human stem cell factor in heavily pretreated patients who have failed a previous attempt with a granulocyte colony-stimulating factor-based regimen. Bone Marrow Transplant 2005; 36: 389–396.
Costa JJ, Demetri GD, Harrist TJ, Dvorak AM, Hayes DF, Merica EA et al. Recombinant human stem cell factor (kit ligand) promotes human mast cell and melanocyte hyperplasia and functional activation in vivo. J Exp Med 1996; 183: 2681–2686.
Richman CM, Weiner RS, Yankee RA . Increase in circulating stem cells following chemotherapy in man. Blood 1976; 47: 1031–1039.
Abrams RA, Johnston-Early A, Kramer C, Minna JD, Cohen MH, Deisseroth AB . Amplification of circulating granulocyte-monocyte stem cell numbers following chemotherapy in patients with extensive small cell carcinoma of the lung. Cancer Res 1981; 41: 35–41.
Stiff PJ, Murgo AJ, Wittes RE, DeRisi MF, Clarkson BD . Quantification of the peripheral blood colony forming unit-culture rise following chemotherapy. Could leukocytaphereses replace bone marrow for autologous transplantation? Transfusion 1983; 23: 500–503.
Duhrsen U, Villeval JL, Boyd J, Kannourakis G, Morstyn G, Metcalf D . Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. Blood 1988; 72: 2074–2081.
Schwartzberg LS, Birch R, Hazelton B, Tauer KW, Lee Jr P, Altemose R et al. Peripheral blood stem cell mobilization by chemotherapy with and without recombinant human granulocyte colony-stimulating factor. J Hematother 1992; 1: 317–327.
Demirer T, Buckner CD, Bensinger WI . Optimization of peripheral blood stem cell mobilization. Stem Cells 1996; 14: 106–116.
de Boer F, Drager AM, Van Haperen MJ, van der Wall E, Kessler F, Huijgens PC et al. The phenotypic profile of CD34-positive peripheral blood stem cells in different mobilization regimens. Br J Haematol 2000; 111: 1138–1144.
Watts MJ, Ings SJ, Leverett D, MacMillan A, Devereux S, Goldstone AH et al. ESHAP and G-CSF is a superior blood stem cell mobilizing regimen compared to cyclophosphamide 1.5 g m−2 and G-CSF for pre-treated lymphoma patients: a matched pairs analysis of 78 patients. Br J Cancer 2000; 82: 278–282.
Moskowitz CH, Bertino JR, Glassman JR, Hedrick EE, Hunte S, Coady-Lyons N et al. Ifosfamide, carboplatin, and etoposide: a highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant-eligible patients with non-Hodgkin's lymphoma. J Clin Oncol 1999; 17: 3776–3785.
Goldschmidt H, Hegenbart U, Haas R, Hunstein W . Mobilization of peripheral blood progenitor cells with high-dose cyclophosphamide (4 or 7 g/m2) and granulocyte colony-stimulating factor in patients with multiple myeloma. Bone Marrow Transplant 1996; 17: 691–697.
Condomines M, Quittet P, Lu ZY, Nadal L, Latry P, Lopez E et al. Functional regulatory T cells are collected in stem cell autografts by mobilization with high-dose cyclophosphamide and granulocyte colony-stimulating factor. J Immunol 2006; 176: 6631–6639.
Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med 2003; 349: 2495–2502.
Pasquini M, Wang Z, Schneider L . Part I—CIBMTR Summary Slides, 2007. CIBMTR Newsletter 2007; 13: 5–8 Available at http://www.cibmtr.org/PUBLICATIONS/Newsletter/index.html. Accessed on 14 January 2007.
Bourhis JH, Bouko Y, Koscielny S, Bakkus M, Greinix H, Derigs G et al. Relapse risk after autologous transplantation in patients with newly diagnosed myeloma is not related with infused tumor cell load and the outcome is not improved by CD34+ cell selection: long term follow-up of an EBMT phase III randomized study. Haematologica 2007; 92: 1083–1090.
Cytoxan (cyclophosphamide) [package insert]. Mead Johnson Oncology Products: Princeton, NJ, 2000.
Kalaycio M, Rybicki L, Pohlman B, Sobecks R, Andresen S, Kuczkowski E et al. Risk factors before autologous stem-cell transplantation for lymphoma predict for secondary myelodysplasia and acute myelogenous leukemia. J Clin Oncol 2006; 24: 3604–3610.
Holtan SG, Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB et al. Timing of autologous stem cell transplantation from last chemotherapy affects lymphocyte collection and survival in non-Hodgkin lymphoma. Br J Haematol 2006; 133: 628–633.
Russell NH, McQuaker G, Stainer C, Byrne JL, Haynes AP . Stem cell mobilisation in lymphoproliferative diseases. Bone Marrow Transplant 1998; 22: 935–940.
Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia 2007; 21: 2035–2042.
Kanfer EJ, McGuigan D, Samson D, Abboudi Z, Abrahamson G, Apperley JF et al. High-dose etoposide with granulocyte colony-stimulating factor for mobilization of peripheral blood progenitor cells: efficacy and toxicity at three dose levels. Br J Cancer 1998; 78: 928–932.
Fruehauf S, Klaus J, Huesing J, Veldwijk MR, Buss EC, Topaly J et al. Efficient mobilization of peripheral blood stem cells following CAD chemotherapy and a single dose of pegylated G-CSF in patients with multiple myeloma. Bone Marrow Transplant 2007; 39: 743–750.
Neulasta (pegfilgrastim) [package insert]. Amgen Inc.: Thousand Oaks, CA, 2007.
Steidl U, Fenk R, Bruns I, Neumann F, Kondakci M, Hoyer B et al. Successful transplantation of peripheral blood stem cells mobilized by chemotherapy and a single dose of pegylated G-CSF in patients with multiple myeloma. Bone Marrow Transplant 2005; 35: 33–36.
Fenk R, Hieronimus N, Steidl U, Bruns I, Graef T, Zohren F et al. Sustained G-CSF plasma levels following administration of pegfilgrastim fasten neutrophil reconstitution after high-dose chemotherapy and autologous blood stem cell transplantation in patients with multiple myeloma. Exp Hematol 2006; 34: 1296–1302.
Matthys P, Hatse S, Vermeire K, Wuyts A, Bridger G, Henson GW et al. AMD3100, a potent and specific antagonist of the stromal cell-derived factor-1 chemokine receptor CXCR4, inhibits autoimmune joint inflammation in IFN-gamma receptor-deficient mice. J Immunol 2001; 167: 4686–4692.
Hatse S, Princen K, Bridger G, De Clercq E, Schols D . Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett 2002; 527: 255–262.
Gerlach LO, Skerlj RT, Bridger GJ, Schwartz TW . Molecular interactions of cyclam and bicyclam non-peptide antagonists with the CXCR4 chemokine receptor. J Biol Chem 2001; 276: 14153–14160.
Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood 2005; 106: 1867–1874.
Calandra G, McCarty J, McGuirk J, Tricot G, Crocker SA, Badel K et al. AMD3100 plus G-CSF can successfully mobilize CD34+ cells from non-Hodgkin's lymphoma, Hodgkin's disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: compassionate use data. Bone Marrow Transplant 2008; 41: 331–338.
DiPersio J, Stadtmauer EA, Nademanee AP, Stiff P, Micallef I, Angell J et al. A phase III, multicenter, randomized, double-blind, placebo controlled, comparative trial of AMD3100 (plerixafor)+G-CSF vs G-CSF+placebo for mobilization in multiple myeloma (MM) patients for autologous hematopoietic stem cell (aHSC) transplantation. Blood (ASH Annual Meeting Abstracts) 2007; 110: 445.
DiPersio JF, Micallef I, Stiff PJ, Bolwell BJ, Maziarz RT, Angell J et al. A phase III, multicenter, randomized, double-blind, placebo controlled, comparative trial of AMD3100 (plerixafor)+G-CSF vs. placebo+G-CSF in non-Hodgkin's lymphoma (NHL) patients for autologous hematopoietic stem cell (aHSC) transplantation. Blood (ASH Annual Meeting Abstracts) 2007; 110: 601.
Micallef I, Stiff PJ, DiPersio JF, Maziarz RT, McCarty JM, Angell J et al. Successful stem cell mobilization rescue by AMD3100 (plerixafor)+G-CSF for patients who failed primary mobilization: results from the phase III (3101-NHL) study. Blood (ASH Annual Meeting Abstracts) 2007; 110: 602.
Somlo G, Sniecinski I, ter Veer A, Longmate J, Knutson G, Vuk-Pavlovic S et al. Recombinant human thrombopoietin in combination with granulocyte colony-stimulating factor enhances mobilization of peripheral blood progenitor cells, increases peripheral blood platelet concentration, and accelerates hematopoietic recovery following high-dose chemotherapy. Blood 1999; 93: 2798–2806.
Linker C, Anderlini P, Herzig R, Christiansen N, Somlo G, Bensinger W et al. Recombinant human thrombopoietin augments mobilization of peripheral blood progenitor cells for autologous transplantation. Biol Blood Marrow Transplant 2003; 9: 405–413.
Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood 2001; 98: 3241–3248.
Basser RL, O'Flaherty E, Green M, Edmonds M, Nichol J, Menchaca DM et al. Development of pancytopenia with neutralizing antibodies to thrombopoietin after multicycle chemotherapy supported by megakaryocyte growth and development factor. Blood 2002; 99: 2599–2602.
Basser R . The impact of thrombopoietin on clinical practice. Curr Pharm Des 2002; 8: 369–377.
Adams GB, Martin RP, Alley IR, Chabner KT, Cohen KS, Calvi LM et al. Therapeutic targeting of a stem cell niche. Nat Biotechnol 2007; 25: 238–243.
Ballen KK, Shpall EJ, Avigan D, Yeap BY, Fisher DC, McDermott K et al. Phase I trial of parathyroid hormone to facilitate stem cell mobilization. Biol Blood Marrow Transplant 2007; 13: 838–843.
Acknowledgements
Friends of Jose Carreras International Leukemia Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bensinger, W., DiPersio, J. & McCarty, J. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant 43, 181–195 (2009). https://doi.org/10.1038/bmt.2008.410
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2008.410
Keywords
This article is cited by
-
Stem cell mobilization in multiple myeloma: challenges, strategies, and current developments
Annals of Hematology (2023)
-
Efficacy of hematopoietic stem cell mobilization regimens in patients with hematological malignancies: a systematic review and network meta-analysis of randomized controlled trials
Stem Cell Research & Therapy (2022)
-
Comparison of the efficacy of hematopoietic stem cell mobilization regimens: a systematic review and network meta-analysis of preclinical studies
Stem Cell Research & Therapy (2021)
-
Nociceptive nerves regulate haematopoietic stem cell mobilization
Nature (2021)
-
New aspects of HSC mobilization for better therapeutic outcomes
Cellular & Molecular Immunology (2021)