Abstract
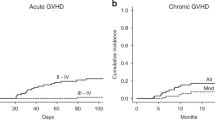
The objective of this study was to determine the efficacy and safety of low-dose recombinant interleukin-2(IL-2) administered to patients with acute lymphoblastic malignancy at high-risk of relapse after unmanipulated HLA-identical or HLA-haploidentical allogeneic hematopoietic stem cell transplantation (allo-HSCT). We studied 19 patients with acute lymphoblastic malignancy who underwent IL-2 treatment for a high probability of disease recurrence after allo-HSCT between July 2004 and June 2006 at Peking University Institute of Hematology. With a median follow-up of 6 months (range, 3–19 months) after the first IL-2 therapy, 14 of 15 evaluable patients in our cohort were disease-free (93.33%), whereas one patient in ‘high risk’ pretransplantation category relapsed. Toxicities from IL-2 were mainly fever, pain, redness and swelling at the injection site. Four patients left the study because of hyperpyrexia. Local and reversible chronic GVHD was observed in 6 of 15 patients (40%). Similar cGVHD occurrences were observed between the two groups of patients undergoing HLA-identical HSCT (three of seven patients) and HLA-haploidentical HSCT (two of six patients), respectively. In conclusion, low-dose IL-2 subcutaneous administration from 100 days for a prolonged period could be a safe and effective strategy to prevent relapse in acute lymphoblastic malignancy patients with high risk of recurrence after unmanipulated allo-HSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990; 75: 555–562.
Guzman ML, Jordan CT . Considerations for targeting malignant stem cells in leukemia. Cancer Control 2004; 11: 97–104.
Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol 1988; 6: 1562–1568.
Weiden PL, Flournoy N, Thomas ED, Fefer A, Buckner CD et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med 1979; 300: 1068–1073.
Slavin S, Ackerstein A, Naparstek E, Or R, Weiss L . The graft-versus-leukemia (GVL) phenomenon: is GVL separable from GVHD? Bone Marrow Transplant 1990; 6: 155–161.
Schleuning M . Adoptive allogeneic immunotherapy-history and future perspectives. Transfus Sci 2000; 23: 133–150.
Sykes M, Harty MW, Szot GL . Interleukin-2 inhibits graft-versus-host disease-promoting activity of CD4+ cells while preserving CD4- and CD8-mediated graft-versus-leukemia effects. Blood 1994; 83: 2560–2569.
Weiss L, Reich S, Slavin S . Effect of cyclosporine A and methylprednisolone on the graft-versus-leukemia effects across major histocompatibility barriers in mice following allogeneic bone marrow transplantation. Bone Marrow Transplant 1990; 6: 229–233.
West WH, Tauer KW, Yannelli JR, Marshall GD, Orr DW, Thurman GB et al. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med 1987; 316: 898–905.
Robinson N, Sanders JE, Benyunes MC, Beach K, Lindgren C, Thompson JA et al. Phase I trial of interleukin-2 after unmodified HLA-matched sibling bone marrow transplantation for children with acute leukemia. Blood 1996; 87: 1249–1254.
Slavin S, Naparstek E, Nagler A, Ackerstein A, Samuel S, Kapelushnik J et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human IL-2 to treat leukemia relapse after allogeneic BMT. Blood 1996; 87: 2195–2204.
Nadala E, Fowlerb A, Kanfera E, Apperley J, Goldman J, Dazzi F . Adjuvant interleukin-2 therapy for patients refractory to donor lymphocyte infusions. Exp Hematol 2004; 32: 218–223.
Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood 2006; 107: 3065–3073.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W et al. Haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 2006; 38: 291–297.
Taniguchi T, Matsui H, Fujita T, Takaoka C, Kashima N, Yoshimoto R et al. Structure and expression of a cloned cDNA for human interleukin-2. Nature 1983; 302: 305–310.
Smith KA . Interleukin-2: inception, impact, and implications. Science 1988; 240: 1169–1176.
Malek TR, Bayer AL . Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol 2004; 4: 665–674.
Nelson BH . IL-2, regulatory T cells, and tolerance. J Immunol 2004; 172: 3983–3988.
Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D et al. IL-2 regulates FOXP3 expression in human CD4+CD25+regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 2006; 108: 1571–1579.
Sullivan KM, Storb R, Buckner CD, Fefer A, Fisher L, Weiden PL et al. Graft-versus-host-disease as adoptive immunotherapy in patients with advanced hematologic neoplasms. N Engl J Med 1989; 320: 828–834.
Marmont AM, Horowitz MM, Gale RP, Sobocinski K, Ash RC, van Bekkum DW et al. T-cell depletion of HLA identical transplants in leukemia. Blood 1991; 78: 2120–2130.
Johnson BD, Truitt RL . Delayed infusion of immunocompetent donor cells after bone marrow transplantation breaks graft-host tolerance and allows for persistent antileukemic reactivity without severe graft-versus-host disease. Blood 1995; 85: 3302–3312.
Billiau AD, Fevery S, Rutgeerts O, Landuyt W, Waer M . Crucial role of timing of donor lymphocyte infusion in generating dissociated graft-versus-host and graft-versus-leukemia responses in mice receiving allogeneic bone marrow transplants. Blood 2002; 100: 1894–1902.
Huang XJ, Liu DH, Xu LP, Chen H, Han W, Liu KY et al. Prophylactic infusion of donor granulocyte colony stimulating factor mobilized peripheral progenitor cells after allogeneic hematopoietic stem cell transplantation in patients with high-risk leukemia. Leukemia 2006; 20: 365–368.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W . Donor lymphocyte infusion for the treatment of leukemia relapse after HLA-mismatched/haploidentical T-cell-replete hematopoietic stem cell transplantation. Haematologica 2007; 92: 414–417.
Acknowledgements
This work was supported by High Technology Research and Development Program of China (No. 2006AA02Z4A0), Key Project Foundation of the Ministry of Public Health and Program for Innovative Research Team in University (No.IRT0702).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, KY., Chen, YH., Liu, DH. et al. A pilot study of low-dose recombinant interleukin-2 for acute lymphoblastic malignancy after unmanipulated allogeneic blood and marrow transplantation. Bone Marrow Transplant 42, 535–539 (2008). https://doi.org/10.1038/bmt.2008.208
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2008.208
Keywords
This article is cited by
-
Preemptive low-dose interleukin-2 or DLI for late-onset minimal residual disease in acute leukemia or myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation
Annals of Hematology (2021)
-
Favorable NK cell activity after haploidentical hematopoietic stem cell transplantation in stage IV relapsed Ewing’s sarcoma patients
Bone Marrow Transplantation (2015)
-
Haploidentical hematopoietic SCT may be superior to conventional consolidation/maintenance chemotherapy as post-remission therapy for high-risk adult ALL
Bone Marrow Transplantation (2015)