Abstract
Background:
We evaluated systemic immune-inflammation index (SII) and its association with patient outcome in germ-cell tumours (GCTs).
Methods:
Two independent cohorts of patients were analysed; the discovery set (n=171) from a single institution and the validation set (n=181) previously included in a study evaluating PD-L1 in GCTs. The SII was calculated using platelet (P), neutrophil (N) and lymphocyte (L) counts before chemotherapy and correlated with survival using regression analyses and Kaplan–Meier method.
Results:
In the discovery cohort, the SII was associated with poor risk clinical features. Patients with low SII had significantly longer progression-free survival (HR=0.22, 95% CI 0.12–0.41, P<0.001) and overall survival (OS) (HR=0.16, 95% CI 0.08–0.32, P<0.001) compared to high SII. This index was independent of International Germ Cell Cancer Collaborative Group criteria in multivariable Cox regression analysis for OS and was validated in an independent cohort. When combining PD-L1 expression on tumour infiltrating lymphocytes (TILs) and SII, we identified three distinctive prognostic groups.
Conclusions:
High SII was associated with poor outcome in GCTs. Combination of PD-L1 positive TILs and SII could further refine prognosis in GCTs.
Similar content being viewed by others
Main
Immune mechanisms have a significant role in antitumour response and cancer development (Mantovani et al, 2008). Testicular germ cell tumours (GCTs) have an exceptional sensitivity to platinum-based chemotherapy (Einhorn, 1979, 1990; Kondagunta et al, 2005; Mardiak et al, 2005; Mead et al, 2005). However, patients who fail front-line and salvage chemotherapy are rendered incurable (Motzer, 2000; Einhorn et al, 2007). Scientific uncertainty regarding mechanisms of resistance to chemotherapy leads to further research in an effort to understand treatment failure in this subset of GCT patients (Romano et al, 2016; Sestakova et al, 2016; Albany et al, 2017). Numerous studies confirmed the efficacy of the check-point inhibition in various types of malignancies in recent years (Topalian et al, 2012; Herbst et al, 2014; Powles et al, 2014; Ansell et al, 2015; Garon et al, 2015). Furthermore, the expression of programmed death receptor 1 ligand (PD-L1) on tumour cells and tumour infiltrating lymphocytes (TILs) has shown a significant prognostic power in our series of patients (Cierna et al, 2016; Chovanec et al, 2017). The inflammatory tumour microenvironment (TME) has many roles in tumour progression and metastasis. Simple blood test such as complete blood count (CBC) can identify immune-inflammatory elements (neutrophils, lymphocytes and platelets) that might shed light on the inflammatory TME (Mantovani et al, 2008; Lippitz, 2013). Immune inflammatory cells have proven to be prognostic in several types of cancer, including urothelial, colorectal, renal cell cancer and mesothelioma (Kishi et al, 2009; Kao et al, 2010; Santoni et al, 2013; Hu et al, 2015; Rossi et al, 2015). Neutrophil to lymphocyte ratio was previously developed as a prognostic tool and was widely tested in other solid tumours (Xiao et al, 2013; Zheng et al, 2013; Balta et al, 2014). Additional value of platelets was suggested, as platelets were shown to protect circulating tumour cells from shear stress during circulation, induce epithelial–mesenchymal transition and promote tumour extravasation to metastatic sites (Labelle et al, 2011; Placke et al, 2012; Schumacher et al, 2013). Neutrophils, lymphocytes, and platelets have been recently used in a joined tool, a systemic immune-inflammation index (SII), to provide prognostic information in patients with malignant tumours (Hu et al, 2014; Lolli et al, 2016a). It was established that SII provides a more powerful tool combining three independent prognostic factors compared to platelets, neutrophil to lymphocytes- or neutrophil to platelets-based tools in cancer (Hong et al, 2015; Wang et al, 2017; Yu et al, 2017). High SII reflects pro-inflammatory activity, which was linked to progression, metastasis and poor outcome in cancer (Seruga et al, 2008; Cools-Lartigue et al, 2013). SII was also associated with higher counts of circulating tumour cells (Hu et al, 2014; Zheng et al, 2017). In this study, we evaluated the prognostic value of SII in GCTs and correlated with PD-L1 expression on tumour cells and TILs.
Patients and methods
This retrospective translational study included a discovery set (DS) of 171 patients with GCTs treated from 1999 to 2015 in the National Cancer Institute in Slovakia, with available CBC before systemic platinum-based chemotherapy and sufficient follow-up clinical data. A validation set (VS) consisted of 181 patients included in our previous translational trial of 240 patients evaluating a prognostic significance of PD-L1 on TILs in GCTs, for whom baseline CBC data and sufficient follow-up clinical data were available (Chovanec et al, 2017). In contrast, the current study aimed to evaluate the prognostic significance of SII and explore its’ associations with PD-L1 expression in GCTs. Clinical data were recorded and compared with SII and PD-L1 expression on TILs. The Institutional Review Board approved this study and a waiver of consent form for patients was granted.
Systemic immune-inflammation index
The SII is an index based on platelets (P), neutrophils (N) and lymphocytes (L) counts. It was calculated using the following formula: SII=P × N/L as defined previously (Hu et al, 2014). The median value was used as the cutoff value of SII, which was then dichotomised into low (below median) and high (above median) categories.
Tumour pathology, tissue microarray construction and immunohistochemical staining for PD-L1
The present study assessed the association of SII with the PD-L1 expression, which was evaluated in 181 of 240 patients in our previous translational study (Chovanec et al, 2017). Tumour specimens collected before the administration of systemic therapy were reviewed by two pathologists associated with the study and PD-L1 expression was evaluated in tumour and on TILs as described previously (Chovanec et al, 2017). Germ-cell tumours were identified according to the World Health Organisation criteria (Moch et al, 2016). Tissue microarray construction and immunohistochemical staining with rabbit anti-PD-L1 monoclonal antibody was described in detail previously (Chovanec et al, 2017). Tumour infiltrating lymphocytes were identified with haematoxylin–eosin staining according to the typical morphology. Tumour cells and TILs with PD-L1 expressions were scored by a weighted histoscore (HS), which accounts for both the extent of cell staining (S) and the staining intensity (I) (Kirkegaard et al, 2006). Positively staining cells were estimated on a scale from 0 to 100%. Subsequently, a score from 0 to 3 (0=no staining; 1=weak; 2=intermediate; and 3=strong staining) was assigned to describe the average intensity of positively staining cells. The HS was then calculated by following formula: S × I; to yield a scale from 0 to 300. On the basis of the HS, a PD-L1 expression was graded as low (0–150) or high (160–300) as we described previously (Mego et al, 2013). The highest PD-L1 expression among GCT subtypes was chosen for mixed GCTs.
Statistical analysis
A Shapiro–Wilk test have shown a significant difference from the normal distribution of PD-L1 HS, therefore non-parametric tests were used for analyses. Differences in distributions of PD-L1 expression between two groups of patients were analysed using the Mann–Whitney U test. For analyses of associations between the SII and PD-L1 expression, a one-way analysis of variance was used when using PD-L1 as a continuous variable. We used Fisher’s exact test to assess the associations between SII and PD-L1 when used as categorical variables.
A median follow-up time was identified as a median time duration since the diagnosis to the time of the last follow-up. Progression-free survival (PFS) was calculated from the date of orchiectomy or tumour biopsy to the date of progression or death or the date of the last adequate follow-up. Overall survival (OS) was calculated from the date of diagnosis to the date of death or last follow-up. We performed a Kaplan–Meier analysis to estimate PFS and OS using a product limit method and and we subsequently compared the results by the log-rank test. A multivariable analysis was performed using Cox proportional hazards model for PFS and OS to assess the differences in prognosis on the basis of SII and PD-L1 expression and a prognosis according to International Germ Cell Cancer Collaborative Group (IGCCCG) criteria (International Germ Cell Cancer Collaborative Group, 1997). All reported P-values were two-sided. A P-value <0.05 was considered as significant. Statistical analyses were performed using NCSS 10 software (NCSS, 2015, LLC. Kaysville, UT, USA, ncss.com/software/ncss).
Results
Patients’ characteristics from discovery and VSs are shown in Table 1. Majority of patients in the discovery and the VS had a non-seminoma histology. A testicular tumour was the most common primary site and more than half of patients were in a good risk category according to the IGCCCG criteria. The median follow-up in the DS was 49 months (0–170) for all patients and 49 months (7–179) for patients still alive. During the follow-up, 42 (25%) patients experienced a disease progression and 34 (20%) patients have died. The estimated 5-year PFS and OS was 75% (95% confidence interval (CI) 68–81%) and 78% (95% CI 73–86%), respectively. The median time of follow-up in the VS was 85 months (0–189) for all patients and 90 months (22–189) for patients still alive. During the follow-up, 42 (25%) of these patients experienced a disease progression and 34 (20%) have died. The estimated 5-year PFS and OS was 89% (95% CI 84–9%) and 91% (95% CI 87–95%), respectively. We attribute the difference in 5-year survival between the DS and the VS to the higher proportion of IGCCCG good-risk patients and lower proportion of IGCCCG poor-risk patients in the VS (Table 1).
Association between the SII and patient/tumour characteristics
We found strongly significant correlations between the SII and poor patients’ characteristics in both cohorts (Table 2). Poor and intermediate risk IGCCCG categories and multiple metastatic sites were associated with the high SII in both groups (all P<0.001). Bulky retroperitoneal disease, liver or other non-visceral pulmonary metastases (NPVM) were also significantly associated with the high SII (all P<0.001). Moreover, the high SII was also associated with high tumour markers (both P<0.001). The SII did not significantly differ between seminomas and non-seminomas, although it was significantly higher in patients with extragonadal primary in the DS but not in the VS (P<0.001 vs 0.238).
A prognostic role of the SII
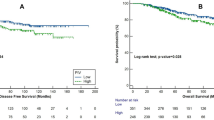
Median SII in DS was 1003. Patients with low SII (SII<1003) had a significantly longer PFS (HR=0.22, 95% CI 0.12–0.41, P<0.001) (Figure 1A) and OS (HR=0.16, 95% CI 0.08–0.32, P<0.001) (Figure 1B) opposite to patients with high SII (SII ⩾1003).
Kaplan–Meier estimates of probabilities of PFS and OS according to the SII. (A) Estimates of probabilities of PFS in the discovery set of patients with GCTs (n=171), HR=0.22, 95% CI 0.12–0.41, P<0.001; low SII<1003; high SII⩾1003. (B) Estimates of probabilities of OS according to the SII; in the discovery set of patients with GCTs (n=171), HR=0.16, 95% CI 0.08–0.32, P<0.001; low SII<1003; high SII⩾1003. CI=confidence interval; GCTs=germ-cell tumours; HR=hazard ratio; OS=overall survival; PFS=progression–free survival; SII=Systemic immune-inflammation index.
A model with median obtained from the discovery data set was tested in an independent VS as defined above. This analysis confirmed prognostic value of SII in GCTs. Patients within the VS, who had a low SII, calculated with a median obtained from the DS had a significantly longer PFS (HR=0.30, 95% CI 0.11–0.81, P=0.004) and OS (HR=0.15, 95% CI 0.05–0.47, P<0.001) (Figure 2) as opposed to patients with the high SII.
Kaplan–Meier estimates of probabilities of PFS and OS according to the SII. (A) Estimates of probabilities of PFS according to the SII; in the validation set of patients with GCTs (n=171), HR=0.30, 95% CI 0.11–0.81, P=0.004; low SII <1003. (B) Estimates of probabilities of OS according to the SII; in the validation set of patients with GCTs (n=171), HR=0.15, 95% CI 0.05–0.47, P<0.001; low SII<1003; high SII ⩾1003. CI=confidence interval; GCTs=germ-cell tumours; HR=hazard ratio; OS=overall survival; PFS=progression-free survival; SII=Systemic immune-inflammation index.
Survival analysis of both study cohorts have reported significantly longer PFS (HR=0.22, 95% CI 0.13–0.37, P<0.0001) and OS (HR=0.14, 95% CI 0.08–0.25, P<0.0001) for patients with low SII compared to patients with high SII (Supplementary Figure 2). A multivariable Cox regression analysis has shown that SII was prognostic independently of IGCCCG for OS, but not for PFS when we compared IGCCCG poor vs good/intermediate-risk patients (Table 3). When we performed the multivariable Cox regression analysis with three IGCCCG categories, the analysis lost the statistical significance for OS (data not shown).
The association of the SII and PD-L1 expression
No statistically significant correlation between the SII and PD-L1 expression on tumour or TILs in the VS was observed. The mean HS for PD-L1 on TILs was 107.3 (95% CI 89.0–125.5) in patients with low SII, compared to 89.1 (95% CI 59.0–119.1) in patients with high SII (P=0.376). Similarly, the mean HS for PD-L1 on tumour cells in patients with low vs high SII was 79.5 (95% CI 66.2–92.8) vs 61.2 (95% CI 41.6–80.8) (P=0.199).
A Fisher’s exact test of the SII and PD-L1 expression on tumour or TILs also reported no significant correlations (P=0.510 and P=0.484, respectively).
A combined prognostic role of the SII and PD-L1 expressing TILs
Previously, we have shown the prognostic value of PD-L1 expressing TILs in GCTs (16). In the subsequent analysis, we assessed a combined prognostic value of SII and PDL-1 on TILs within the VS. The analysis identified three prognostic groups of patients. The best prognosis was seen in patients who had a high expression of PD-L1 on TILs (HS ⩾160) and a low SII (SII<1003) with a 5-year PFS and OS of both 100%, while the worst prognosis was seen in patients with a low expression of PDL1 on TILs (HS <160) and a high SII (SII⩾1003) with a 5-year PFS and OS of 70% and 70%, respectively (HR=0.29, 95% CI 0.10–0.78; P<0.001 for PFS and HR=0.13, 95% CI 0.04–0.40; P<0.001 for OS) (Supplementary Figure 1). Patients with SII and PD-L1 on TILs both low or high had similar intermediate prognosis.
Discussion
Immune mechanisms have been associated with the pathogenesis of cancer (Sharma and Allison, 2015). Recent advancements in anticancer treatment with immune therapy unleashing the immunity of the host and driving the anti-tumour response resulted in long-term remissions, and even cure in several malignancies (Brahmer et al, 2015; Larkin et al, 2015; Motzer et al, 2015). Testicular cancer has been traditionally referred to as chemotherapy sensitive and few facts are known about the underlying immune mechanisms in this disease. In this study, we analysed a prognostic value of a SII in two independent retrospective cohorts of patients and its association with PD-L1 expression on TILs in our VS. We found that SII calculated prior to chemotherapy is an indicator of prognosis among GCT patients. We did not observe correlation between SII and PD-L1 expression in tumour cells and/or PD-L1 expressing TILs, but the combination of SII and PD-L1 on TILs have created a robust prognostic tool for clinical outcome in GCTs. Our results suggest that immune processes have a role in the mechanisms of progression in GCTs, however, based on our data we cannot determine whether systemic inflammation as expressed by SII creates a permissive microenvironment that leads to the manifestation of the disease with poor prognostic features or if the SII reflects an aggressive disease. However, the ability to predict the clinical outcome using the host’s immune parameters could allow the pre-selection of patients with different prognostic profiles and the consequent planning of tailored treatment. Interestingly, Yuksel et al (2016) recently reported a neutrophil-to-lymphocyte ratio as a simple marker predictive of the presence of stage I testicular cancer. However, Bolat et al, (2017) evaluated a prognostic significance of pre-orchiectomy neutrophil-to-lymphocyte ratio in GCT patients and observed no difference in PFS and cancer-specific survival. A revised version of the IGCCCG classification (28) has been launched in 2016, which is collecting data also on P, N and L at baseline in patients treated with first-line chemotherapy (Collette, 2017). This large series of thousands of cases could contribute to better understanding the impact of these parameters including the SII in GCTs.
A cytokine signalling suggesting pro-inflammatory and immunosuppressive pathways that predicts prognosis in GCTs has been previously described in our works (Chovanec et al, 2015; Svetlovska et al, 2017). Pro-inflammatory TME has been reported to correspond with poor prognosis in other malignancies as well (Li et al, 2014; Tsai et al, 2014, 2017). However, the immune TME and its impact on outcome in patients with GCTs is not entirely clear. The SII in our DS have shown strong correlations with essentially all poor clinical characteristics in GCTs. All correlations with clinical features such as the extra-gonadal primary, bulky retroperitoneal disease, NPVM or elevated tumour markers, were strongly significant. We have demonstrated the prognostic value of SII in GCTs for the first time, evidenced by a significant difference in PFS and OS. These findings were replicated in the independent VS. While all the poor clinical characteristics were significantly associated high SII, similar to the data from the DS, one exception was seen. Only two patients were categorised as having primary extra-gonadal tumour within the VS, which contributed to the low statistical power in this tumour characteristic. In a study by Lolli et al (2016b), a higher SII was also associated with poor outcome and poor clinical features, such as Gleason score ⩾8, visceral metastases or ECOG ⩾2, in patients with prostate cancer. Similar data were reported by the same group and others in kidney cancer, oesophageal squamous, hepatocellular, gastric or colorectal carcinoma (Huang et al, 2016; Passardi et al, 2016; Wang et al, 2016; Lolli et al, 2016a; Feng et al, 2017). With the recent development of immune therapies, PD-1, PD-L1 and CTLA4 have become treatment targets in cancer. Germ-cell tumours express PD-L1 in abundance, as was shown by Fankhauser and our group previously (Fankhauser et al, 2015; Cierna et al, 2016). In addition, PD-L1 but not PD-1 expression was prognostic when expressed on tumour cells and TILs (Cierna et al, 2016; Chovanec et al, 2017). A phase II clinical study from Indiana University evaluating an anti-PD-1 agent pembrolizumab failed to prove an efficacy in the treatment of refractory GCTs (Adra et al, 2017). Initial results in seven cases with platinum-refractory GCTs treated with anti-PD1 agents (pembrolizumab or nivolumab) after high-dose chemotherapy have been recently reported with possible activity in three patients (Zschabitz et al, 2016, 2017) and a single case report provided evidence of ongoing partial remission with marker stabilisation with nivolumab (Chi and Schweizer, 2017). However, predictive markers associated with tumour response have not been reported and larger prospective clinical trials are suggested. The associations of inflammatory pathways in relation with PD-1/PD-L1 signalling is unknown. Our further investigation of the association of SII and PD-L1 on tumour cells and TILs did not confirm significant correlations among these, suggesting that PD-L1 and SII mirror different aspects of immunity. An analysis of a combined prognostic value of the SII and PD-L1 on TILs in our VS have discovered three groups of patients; (i) with an excellent prognosis (no events reported) if PD-L1 on TILs was high and SII was low, (ii) a poor prognosis if PD-L1 on TILs was low and SII was high, (iii) and an intermediate prognosis if both were low or high. While the survival curves showed smaller differences in patients with better outcomes for OS compared to PFS in this survival analysis, no events were observed in the best prognostic group (100% PFS and 100% OS at 14 years (Supplementary Figure 1). This is an interesting observation, as well established IGCCCG prognostic criteria show 90% 5-year PFS and 97% 5-year OS for good risk patients (1997). Therefore, combined SII and PD-L1 seems to provide more prognostic power compared to the IGCCCG and may be a useful tool for prognosis prediction in the future. We speculate that the favourable prognosis that might be driven by PD-L1 on TILs can be reduced in the pro-inflammatory environment and vice-versa. The underlying mechanism is however unclear and more research is needed to provide a detailed explanation.
Our study have some strengths and limitations. The strength of the study is the existence of DS and VS as well as the size of the patient population. Limitations include the retrospective nature of the analysis and under-representation of extragonadal GCTs. Also, we noted some imbalances between the sets that could be responsible for differences in the median of SII. While the most of patients in both cohorts were in IGCCCG good risk, the VS included less poor risk and extragonadal GCTs than the DS, which could explain lower overall SII index in the VS compared to the DS.
In conclusion, this is the first translational study to show a prognostic potency of SII in GCTs. On the basis of the acquired data, we suggest SII and its’ combined prognostic value with PD-L1 as an interesting novel finding that requires further larger and prospective study for validation and implementation into clinical practice. Additional research is needed to provide detailed insights into immunobiology of GCTs and uncover possible implications for treatment of this disease.
Change history
20 March 2018
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Adra N, Einhorn LH, Althouse SK, Ammakkanavar NR, Musapatika D, Albany C, Vaughn D, Hanna NH (2017) Phase II trial of pembrolizumab in patients with platinum refractory germ cell tumors: a Hoosier Cancer Research Network study GU14-206. Ann Oncol (pii): 4555286.
Albany C, Hever-Jardine MP, von Herrmann KM, Yim CY, Tam J, Warzecha JM, Shin L, Bock SE, Curran BS, Chaudhry AS, Kim F, Sandusky GE, Taverna P, Freemantle SJ, Christensen BC, Einhorn LH, Spinella MJ (2017) Refractory testicular germ cell tumors are highly sensitive to the second generation DNA methylation inhibitor guadecitabine. Oncotarget 8 (2): 2949–2959.
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 372 (4): 311–319.
Balta S, Demirkol S, Kucuk U, Cakar M, Unlu M (2014) Neutrophil-to-lymphocyte ratio as a novel independent prognostic factor in urothelial carcinoma. Clin Genitourin Cancer 12 (2): e69–e70.
Bolat D, Aydogdu O, Polat S, Yarimoglu S, Bozkurt IH, Yonguc T, Sen V (2017) Predictive value of preoperative neutrophil-to-lymphocyte ratio on the prognosis of germ cell testicular tumors. Turk J Urol 43 (1): 55–61.
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373 (2): 123–135.
Chi EA, Schweizer MT (2017) Durable response to immune checkpoint blockade in a platinum-refractory patient with nonseminomatous germ cell tumor. Clin Genitourin Cancer 15 (5): e855–e857.
Chovanec M, Cierna Z, Miskovska V, Machalekova K, Svetlovska D, Kalavska K, Rejlekova K, Spanik S, Kajo K, Babal P, Mardiak J, Mego M (2017) Prognostic role of programmed-death ligand 1 (PD-L1) expressing tumor infiltrating lymphocytes in testicular germ cell tumors. Oncotarget 8 (13): 21794–21805.
Chovanec M, Mego M, Cholujova D, Gronesova P, Miskovska V, Sycova-Mila Z, Usakova V, Svetlovska D, Bujdak P, Spanik S, Ondrus D, Mardiak J (2015) A cytokine and angiogenic factor (CAF) analysis in plasma in testicular germ cell tumor patients (TGCTs). J Clin Oncol 33 (15): e15552.
Cierna Z, Mego M, Miskovska V, Machalekova K, Chovanec M, Svetlovska D, Hainova K, Rejlekova K, Macak D, Spanik S, Ondrus D, Kajo K, Mardiak J, Babal P (2016) Prognostic value of programmed-death-1 receptor (PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann Oncol 27 (2): 300–305.
Collette L (2017) Update of the international prognostic classification for first line metastatic germ-cell cancers. An international initiative. Eur J Cancer 72 (suppl 1): S196–S197.
Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L (2013) Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest (pii): 67484.
Einhorn LH (1979) Combination chemotherapy with cis-dichlorodiammineplatinum(II) in disseminated testicular cancer. Cancer Treat Rep 63 (9–10): 1659–1662.
Einhorn LH (1990) Treatment of testicular cancer: a new and improved model. J Clin Oncol 8 (11): 1777–1781.
Einhorn LH, Williams SD, Chamness A, Brames MJ, Perkins SM, Abonour R (2007) High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Med 357 (4): 340–348.
Fankhauser CD, Curioni-Fontecedro A, Allmann V, Beyer J, Tischler V, Sulser T, Moch H, Bode PK (2015) Frequent PD-L1 expression in testicular germ cell tumors. Br J Cancer 113 (3): 411–413.
Feng JF, Chen S, Yang X (2017) Systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Medicine 96 (4): e5886.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, Investigators K (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372 (21): 2018–2028.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515 (7528): 563–567.
Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q (2015) Systemic Immune-inflammation Index, Based on Platelet Counts and Neutrophil-Lymphocyte Ratio, Is Useful for Predicting Prognosis in Small Cell Lung Cancer. Tohoku J Exp Med 236 (4): 297–304.
Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, Fan J (2014) Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 20 (23): 6212–6222.
Hu K, Lou L, Ye J, Zhang S (2015) Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: a meta-analysis. BMJ Open 5 (4): e006404.
Huang L, Liu S, Lei Y, Wang K, Xu M, Chen Y, Liu B, Chen Y, Fu Q, Zhang P, Qin K, Cai Y, Fu S, Ge S, Yuan X (2016) Systemic immune-inflammation index, thymidine phosphorylase and survival of localized gastric cancer patients after curative resection. Oncotarget 7 (28): 44185–44193.
International Germ Cell Cancer Collaborative Group (1997) International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 15 (2): 594–603.
Kao SC, Pavlakis N, Harvie R, Vardy JL, Boyer MJ, van Zandwijk N, Clarke SJ (2010) High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res 16 (23): 5805–5813.
Kirkegaard T, Edwards J, Tovey S, McGlynn LM, Krishna SN, Mukherjee R, Tam L, Munro AF, Dunne B, Bartlett JM (2006) Observer variation in immunohistochemical analysis of protein expression, time for a change? Histopathology 48 (7): 787–794.
Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN (2009) Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol 16 (3): 614–622.
Kondagunta GV, Bacik J, Donadio A, Bajorin D, Marion S, Sheinfeld J, Bosl GJ, Motzer RJ (2005) Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol 23 (27): 6549–6555.
Labelle M, Begum S, Hynes RO (2011) Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20 (5): 576–590.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373 (1): 23–34.
Li G, Wang Z, Ye J, Zhang X, Wu H, Peng J, Song W, Chen C, Cai S, He Y, Xu J (2014) Uncontrolled inflammation induced by AEG-1 promotes gastric cancer and poor prognosis. Cancer Res 74 (19): 5541–5552.
Li TJ, Jiang YM, Hu YF, Huang L, Yu J, Zhao LY, Deng HJ, Mou TY, Liu H, Yang Y, Zhang Q, Li GX (2017) Interleukin-17-producing neutrophils link inflammatory stimuli to disease progression by promoting angiogenesis in gastric cancer. Clin Cancer Res 23 (6): 1575–1585.
Lippitz BE (2013) Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 14 (6): e218–e228.
Lolli C, Basso U, Derosa L, Scarpi E, Sava T, Santoni M, Crabb SJ, Massari F, Aieta M, Conteduca V, Maruzzo M, La Russa F, Wheater M, Berardi R, Galli L, De Giorgi U (2016a) Systemic immune-inflammation index predicts the clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Oncotarget 7 (34): 54564–54571.
Lolli C, Caffo O, Scarpi E, Aieta M, Conteduca V, Maines F, Bianchi E, Massari F, Veccia A, Chiuri VE, Facchini G, De Giorgi U (2016b) Systemic immune-inflammation index predicts the clinical outcome in patients with mCRPC treated with abiraterone. Front Pharmacol 7: 376.
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454 (7203): 436–444.
Mardiak J, Salek T, Sycova-Mila Z, Obertova J, Hlavata Z, Mego M, Reckova M, Koza I (2005) Paclitaxel plus ifosfamide and cisplatin in second-line treatment of germ cell tumors: a phase II study. Neoplasma 52 (6): 497–501.
Mead GM, Cullen MH, Huddart R, Harper P, Rustin GJ, Cook PA, Stenning SP, Mason M, Party MRCTTW (2005) A phase II trial of TIP (paclitaxel, ifosfamide and cisplatin) given as second-line (post-BEP) salvage chemotherapy for patients with metastatic germ cell cancer: a medical research council trial. Br J Cancer 93 (2): 178–184.
Mego M, Cierna Z, Svetlovska D, Macak D, Machalekova K, Miskovska V, Chovanec M, Usakova V, Obertova J, Babal P, Mardiak J (2013) PARP expression in germ cell tumours. J Clin Pathol 66 (7): 607–612.
Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs-part a: renal, penile, and testicular tumours. Eur Urol 70 (1): 93–105.
Motzer RJ (2000) Paclitaxel (Taxol) combination therapy for resistant germ cell tumors. Semin Oncol 27 (1 Suppl 1): 33–35.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate I (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373 (19): 1803–1813.
Passardi A, Scarpi E, Cavanna L, Dall'Agata M, Tassinari D, Leo S, Bernardini I, Gelsomino F, Tamberi S, Brandes AA, Tenti E, Vespignani R, Frassineti GL, Amadori D, De Giorgi U (2016) Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget 7 (22): 33210–33219.
Placke T, Salih HR, Kopp HG (2012) GITR ligand provided by thrombopoietic cells inhibits NK cell antitumor activity. J Immunol 189 (1): 154–160.
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515 (7528): 558–562.
Romano FJ, Rossetti S, Conteduca V, Schepisi G, Cavaliere C, Di Franco R, La Mantia E, Castaldo L, Nocerino F, Ametrano G, Cappuccio F, Malzone G, Montanari M, Vanacore D, Quagliariello V, Piscitelli R, Pepe MF, Berretta M, D'Aniello C, Perdona S, Muto P, Botti G, Ciliberto G, Veneziani BM, De Falco F, Maiolino P, Caraglia M, Montella M, De Giorgi U, Facchini G (2016) Role of DNA repair machinery and p53 in the testicular germ cell cancer: a review. Oncotarget 7 (51): 85641–85649.
Rossi L, Santoni M, Crabb SJ, Scarpi E, Burattini L, Chau C, Bianchi E, Savini A, Burgio SL, Conti A, Conteduca V, Cascinu S, De Giorgi U (2015) High neutrophil-to-lymphocyte ratio persistent during first-line chemotherapy predicts poor clinical outcome in patients with advanced urothelial cancer. Ann Surg Oncol 22 (4): 1377–1384.
Santoni M, De Giorgi U, Iacovelli R, Conti A, Burattini L, Rossi L, Luca Burgio S, Berardi R, Muzzonigro G, Cortesi E, Amadori D, Cascinu S (2013) Pre-treatment neutrophil-to-lymphocyte ratio may be associated with the outcome in patients treated with everolimus for metastatic renal cell carcinoma. Br J Cancer 109 (7): 1755–1759.
Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S (2013) Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 24 (1): 130–137.
Seruga B, Zhang H, Bernstein LJ, Tannock IF (2008) Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 8 (11): 887–899.
Sestakova Z, Kalavska K, Hurbanova L, Jurkovicova D, Gursky J, Chovanec M, Svetlovska D, Miskovska V, Obertova J, Palacka P, Rejlekova K, Sycova-Mila Z, Cingelova S, Spanik S, Mardiak J, Chovanec M, Mego M (2016) The prognostic value of DNA damage level in peripheral blood lymphocytes of chemotherapy-naive patients with germ cell cancer. Oncotarget 7 (46): 75996–76005.
Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science 348 (6230): 56–61.
Svetlovska D, Miskovska V, Cholujova D, Gronesova P, Cingelova S, Chovanec M, Sycova-Mila Z, Obertova J, Palacka P, Rajec J, Kalavska K, Usakova V, Luha J, Ondrus D, Spanik S, Mardiak J, Mego M (2017) Plasma cytokines correlated with disease characteristics, progression-free survival, and overall survival in testicular germ-cell tumor patients. Clin Genitourin Cancer 15 (3): 411–416.e2.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366 (26): 2443–2454.
Tsai CY, Wang CS, Tsai MM, Chi HC, Cheng WL, Tseng YH, Chen CY, Lin CD, Wu JI, Wang LH, Lin KH (2014) Interleukin-32 increases human gastric cancer cell invasion associated with tumor progression and metastasis. Clin Cancer Res 20 (9): 2276–2288.
Wang BL, Tian L, Gao XH, Ma XL, Wu J, Zhang CY, Zhou Y, Guo W, Yang XR (2016) Dynamic change of the systemic immune inflammation index predicts the prognosis of patients with hepatocellular carcinoma after curative resection. Clin Chem Lab Med 54 (12): 1963–1969.
Wang K, Diao F, Ye Z, Zhang X, Zhai E, Ren H, Li T, Wu H, He Y, Cai S, Chen J (2017) Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Chin J Cancer 36 (1): 75.
Xiao GQ, Liu C, Liu DL, Yang JY, Yan LN (2013) Neutrophil-lymphocyte ratio predicts the prognosis of patients with hepatocellular carcinoma after liver transplantation. World J Gastroenterol 19 (45): 8398–8407.
Yu J, Wu X, Yu H, Li S, Mao L, Chi Z, Si L, Sheng X, Cui C, Dai J, Ma M, Tang H, Xu T, Yan J, Kong Y, Guo J (2017) Systemic immune-inflammation index and circulating T-cell immune index predict outcomes in high-risk acral melanoma patients treated with high-dose interferon. Transl Oncol 10 (5): 719–725.
Yuksel OH, Verit A, Sahin A, Urkmez A, Uruc F (2016) White blood cell counts and neutrophil to lymphocyte ratio in the diagnosis of testicular cancer: a simple secondary serum tumor marker. Int Braz J Urol 42 (1): 53–59.
Zheng L, Zou K, Yang C, Chen F, Guo T, Xiong B (2017) Inflammation-based indexes and clinicopathologic features are strong predictive values of preoperative circulating tumor cell detection in gastric cancer patients. Clin Transl Oncol 19 (9): 1125–1132.
Zheng YB, Zhao W, Liu B, Lu LG, He X, Huang JW, Li Y, Hu BS (2013) The blood neutrophil-to-lymphocyte ratio predicts survival in patients with advanced hepatocellular carcinoma receiving sorafenib. Asian Pac J Cancer Prev 14 (9): 5527–5531.
Zschabitz S, Lasitschka F, Hadaschik B, Hofheinz RD, Jentsch-Ullrich K, Gruner M, Jager D, Grullich C (2017) Response to anti-programmed cell death protein-1 antibodies in men treated for platinum refractory germ cell cancer relapsed after high-dose chemotherapy and stem cell transplantation. Eur J Cancer 76: 1–7.
Zschabitz S, Lasitschka F, Jager D, Grullich C (2016) Activity of immune checkpoint inhibition in platinum refractory germ-cell tumors. Ann Oncol 27 (7): 1356–1360.
Acknowledgements
We would like to acknowledge our collaborators from departments of pathology in Slovakia: Antol M, Benko J, Danis D, Durcansky D, Fiala P, Galbavy S, Gogora M, Hudcovsky P, Macuch J, Martanovic P, Ondrias F, Plank L and Svajdler M. We would like to acknowledge Theodore Logan for the critical input, Mrs Daniela Jantekova, from the Population Registry of Slovak Republic for updating patients’ database, Mrs Zlatica Pekova for administration support, and Emilia Klincova and Ludovit Gaspar for excellent technical assistance. This work was supported by the Slovak Research and Development Agency under contract APVV-15-0086.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Note: this study was presented on 3–7 June 2017 at the American Society of Clinical Oncology (ASCO) annual meeting, Chicago, IL, USA, as publication-only abstract no. e16042.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Chovanec, M., Cierna, Z., Miskovska, V. et al. Systemic immune-inflammation index in germ-cell tumours. Br J Cancer 118, 831–838 (2018). https://doi.org/10.1038/bjc.2017.460
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2017.460
Keywords
This article is cited by
-
Habitual physical activity modulates cardiometabolic health in long-term testicular cancer survivors
Supportive Care in Cancer (2023)
-
A nomogram combining plasma fibrinogen and systemic immune‑inflammation index predicts survival in patients with resectable gastric cancer
Scientific Reports (2021)
-
Recent Advances and Future Directions of Diagnostic and Prognostic Prediction Models in Ovarian Cancer
Journal of Shanghai Jiaotong University (Science) (2021)
-
Attenuated Salmonella engineered with an apoptosis-inducing factor (AIF) eukaryotic expressing system enhances its anti-tumor effect in melanoma in vitro and in vivo
Applied Microbiology and Biotechnology (2020)
-
Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer
Journal of Translational Medicine (2019)