Abstract
Background:
Body composition is an important predictor of drug toxicity and outcome. Ipilimumab (Ipi), a monoclonal antibody used to treat metastatic melanoma, has specific toxicities. No validated biomarkers that predict Ipi toxicity and efficacy exist. Also, the impact of Ipi on body composition has not been established.
Methods:
Patients with metastatic melanoma treated with Ipi between 2009 and 2015 were included. Body composition was assessed by computed tomography at baseline and after four cycles of Ipi. Sarcopenia and low muscle attenuation (MA) were defined using published cut-points. All adverse events (AEs) and immune-related AEs (irAEs) were recorded (Common Terminology Criteria For Adverse Event V.4.0).
Results:
Eighty-four patients were included in this study (62% male, median age 54 years). At baseline, 24% were sarcopenic and 33% had low MA. On multivariate analysis, sarcopenia and low MA were significantly associated with high-grade AEs (OR=5.34, 95% CI: 1.15–24.88, P=0.033; OR=5.23, 95% CI: 1.41–19.30, P=0.013, respectively), and low MA was associated with high-grade irAEs (OR=3.57, 95% CI: 1.09–11.77, P=0.036). Longitudinal analysis (n=59) revealed significant reductions in skeletal muscle area (SMA), total body fat-free mass, fat mass (all P<0.001) and MA (P=0.030). Mean reduction in SMA was 3.3%/100 days (95% CI: −4.48 to −1.79%, P<0.001). A loss of SMA ⩾7.5%/100 days (highest quartile) was a significant predictor of overall survival in multivariable Cox regression analysis (HR: 2.1, 95% CI: 1.02–4.56, P=0.046).
Conclusions:
Patients with sarcopenia and low MA are more likely to experience severe treatment-related toxicity to Ipi. Loss of muscle during treatment was predictive of worse survival. Treatments to increase muscle mass and influence outcome warrant further investigation.
Similar content being viewed by others
Main
In Europe, malignant melanoma of the skin is the seventh and tenth most common cancer diagnosed in women and men, respectively (International Agency for Research on Cancer, Cancer Fact Sheets 2012). Traditionally, patients with metastatic melanoma have been known to have a dismal prognosis, with a median overall survival of 8–10 months and a 5-year survival rate of 10% (Garbe et al, 2011). Curative surgical options are available in the early stages of the disease; however, once the disease has progressed to an advanced stage, treatment options are largely confined to systemic therapy and long-term remission is uncommon.
In the modern era, due in part to advances in therapies that stimulate an immune-mediated antitumour response, more treatment options are available for those with advanced melanoma. Ipilimumab, a novel anticytotoxic T-cell lymphocyte-4 monoclonal antibody, was the first of its kind to demonstrate a significant survival benefit in previously treated metastatic melanoma patients (median OS 10.1 months vs 6.4 months in gp100 vaccine group; 1- and 2-year survival rates of 45.6% and 23.5%, respectively, in the ipilimumab arm) (Hodi et al, 2010). Following the release of this phase III data, ipilimumab was approved by the US Food and Drug Administration and by the European Medicines Association at a dose of 3 mg kg−1 body weight in patients with metastatic melanoma.
Ipilimumab augments T-cell activation and proliferation with the goal of reducing immune tolerance to cancer-specific antigens (Robert and Ghiringhelli, 2009). As a result, ipilimumab can result in an activation of immune responses against normal tissue, which can result in ‘immune-related adverse events (irAEs)’ (Hodi et al, 2010). The irAEs have been well described. Approximately 61% of patients treated with ipilimumab experience an irAE (any grade), whereas 17% of patients experience a high-grade irAE (grades III and IV) (Bertrand et al, 2015). IrAEs most commonly affect the gastrointestinal (GI) tract, the skin or endocrine glands (Hodi et al, 2010; Bertrand et al, 2015). Such adverse effect can be treated effectively with immunosuppressive agents (Weber et al, 2012). To date, little is known in relation to factors predicting ipilimumab toxicity.
Over the past decade, sarcopenia (low muscle mass) and its relationship to adverse clinical outcomes has been extensively studied and shown to be associated with increase length of hospital stay (Pichard et al, 2004), postoperative infections (Lieffers et al, 2012), functional impairment and decreased survival in both malignant (Tan et al, 2009; Martin et al, 2013) and non-malignant (Montano-Loza et al, 2012) populations. Sarcopenia and its relationship to anticancer treatment toxicity has been particularly topical in the literature, and studies have consistently demonstrated that sarcopenia is associated with poorer treatment tolerance and increased prevalence of dose-limiting toxicity (DLT) to many anticancer/chemotherapeutic drugs, for example, epirubicin (Prado et al, 2011), capecitabine (Prado et al, 2009), sunitinub (Huillard et al, 2013; Cushen et al, 2014), 5-FU and leucovorin (Prado et al, 2007). More recently, low muscle attenuation (MA), which refers to a poor quality skeletal muscle (increased intramuscular adipose tissue) has been the focus of much research. Low MA has been identified as a predictor of reduced survival in renal cell carcinoma (Antoun et al, 2013b) and stage III melanoma (Sabel et al, 2011), as well as lung and GI malignancies (Martin et al, 2013; Blauwhoff-Buskermolen et al, 2016).
To our knowledge, the association between altered/abnormal body composition, that is, sarcopenia and low MA, and the incidence of treatment-related AEs to ipilimumab has not yet been reported. With increasing use of ipilimumab in clinical practice, there is an urgent need for potential biomarkers that are predictive of treatment toxicity. The primary aim of this study was to investigate if body composition, specifically sarcopenia and low MA, assessed by computed tomography (CT) could predict toxicity to ipilimumab in patients with metastatic melanoma. A secondary aim of this study was to determine the changes in body composition that occur during treatment and to assess if these changes impact on survival.
Materials and methods
Study population
We performed a retrospective medical record review of all consecutive adult patients treated with ipilimumab at two university teaching hospitals between the years of 2009 and 2015. Patients were excluded from this study if they lacked an evaluable pre-treatment CT image. This study was approved by the local ethics committee and conducted according to good clinical practice and applicable laws.
Clinical details
Clinical and pathological data was collected by a retrospective chart review, including information on patient demographics, primary tumour site/stage, the extent of metastatic disease, biochemistry results and oncological treatment. Patient’s date of death (if present) or date of last follow-up was recorded.
Anthropometry and body composition
Weight and height closest to the initiation of cycle 1 of treatment was recorded from the medical charts. Body mass index (BMI) was calculated (weight (kg) height (m−2)).
Pre- and post-treatment CT images taken as part of routine patient care were accessed from the hospitals electronically stored database. Body composition was evaluated using CT images, as described previously (Heymsfield et al, 1997). The third lumbar vertebrae (L3) was chosen as the standard landmark (Shen et al, 2004) and two consecutive transverse CT images where both transverse processes were clearly visible were analysed using the OsiriX software version 4.1.1 (Pixmeo, Geneva, Switzerland) and the average result reported. Different tissue compartments were manually outlined and segmentation of the skeletal muscle and adipose tissue was based on Hounsfield unit (HU) thresholds (−29 to +150, and −30 to −190 HU, respectively (Mitsiopoulos et al, 1998). Cross-sectional areas (cm2) for muscle and adipose tissue were automatically calculated by summing tissue pixels and multiplying by pixel surface area after applying HU thresholds. Mean MA is reported for the entire muscle area at L3. Anonymised CT images were analysed by the two trained study assessors (LD and SC) who were blinded to the order of images (pre- and post-ipilimumab treatment). Pre-treatment images were taken before treatment administration (mean 39±31 days). Post-treatment images were taken after the final dose of ipilimumab was administered (mean 35±31 days). The mean number of days between the two scans was 146±40 days. To account for variation in the exact duration of scan intervals, changes in tissue are expressed as % change/100 days to provide a standard measure for all patients.
The lumbar cross-sectional areas of muscle and fat are linearly related to whole-body measures (Shen et al, 2004). Estimates of whole-body fat mass (FM) and fat-free mass (FFM) were calculated using published regression equations as follows (Mourtzakis et al, 2008): FFM (kg)=0.3 × (skeletal muscle cross-sectional area at L3 (cm2))+6.06; r2=0.88, FM (kg)=0.042 × (Adipose tissue cross-sectional area at L3 (cm2))+11.2; r2=0.77. Gender- and BMI-specific cut-points were used to define sarcopenia and low MA (Martin et al, 2013).
Treatment tolerability
Toxicity profiles were recorded on all patients by reviewing patient’s medical notes. Toxicity was recorded across all administered cycles of ipilimumab. The standard dose for ipilimumab is 3 mg kg−1 body weight administered intravenously over a 90-min period every 3 weeks for four doses. Adverse events were classified according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. For further analyses, toxicity was divided into grades I–II and grades III–IV. An irAE was defined as an AE that was associated with exposure to ipilimumab and that was consistent with an immune-related phenomenon. The irAEs recorded were those most commonly reported. A high-grade AE/irAE was defined as a grade III–IV toxicity. A high-grade AE included all high-grade AEs, both general and immune-related high-grade AEs. Any dose delays or early cessation of treatment as a result of significant toxicity (grades III–IV) was recorded, and in this study it was defined as a DLT.
Statistical assessment
Statistical analysis was completed using SPSS (version 21.0, SPSS Inc., Chicago, IL, USA). Data are expressed as mean±s.d. or median (IQR) where appropriate.
Comparisons between groups of patients were assessed using χ2 test for categorical variables and unpaired t-tests and Mann–Whitney U-tests to test for differences between continuous variables. Paired t-tests were used to assess changes in body composition. The McNemar’s test was to test for significances in paired categorical data.
Logistic regression analyses were used to test associations between treatment toxicity and measures of body composition (baseline sarcopenia vs not sarcopenic and low MA vs without low MA). Variables that had significance of P⩽0.25 on univariate analysis were eligible for inclusion in multivariate analysis.
Survival curves were constructed using the Kaplan–Meier technique and log-rank test was used to compare survival between groups of patients. Survival was measured from the date of the baseline (pre-treatment) CT image until the date of death or censored date (study completion). Median follow-up time (time in study) for the entire cohort was 12.8 months [IQR: 5.45–21.7 months; 9.2 months (IQR 4.8–15.3 months) for patients who had died and 24.1 months for censored cases (IQR 15.5–33.4 months)] and 70% of patients had died by completion of the study. Cox proportional hazards analyses was performed, and hazard ratios (HR) with 95% confidence intervals (CI) calculated. All P-values were two sided, and the level of significance was P<0.05.
Results
Participants
From July 2010 to July 2015, 96 patients received ipilimumab, of which 84 patients met the criteria for study analysis (12 patients lacked an evaluable CT image). MA analysis was carried out on all patients with a contrast-enhanced CT image (n=72).
Patient characteristics
Baseline characteristics are presented in Table 1. In brief, among the 84 patients studied, 62% were male with a median age of 54 years (IQR 43–66 years). The majority of patients (79%) had stage M1c metastatic disease, with the most common metastatic sites being lung (70%) and liver (41%). Ipilimumab was first-line treatment in 34.5% of patients.
Anthropometry
Table 2 describes the body composition features of the cohort. Fifty-nine patients (70%) were considered overweight or obese (BMI ⩾25 kg m−2), while very few (only two patients) were clinically underweight by WHO standards. Twenty patients (24%) were sarcopenic (20 of 84) and 24 (24 of 72) (33.3%) were considered to have a low MA.
Sarcopenic patients differed significantly from non-sarcopenic patients as expected with a lower skeletal muscle area (SMA) (161.2 vs 121.4 cm2, P⩽0.001), skeletal muscle index (SMI) (54.7 vs 41.1 cm2 m−2, P<0.001) and FFM (54.4 vs 42.5 kg, P<0.001). Sarcopenic patients had a lower mean MA (41.2 vs 34.7 HU) (P=0.008), and a higher prevalence of those with low MA (64.7% vs 23.6%, P=0.004). A trend was observed that sarcopenic patients were older (60 vs 53 years, P=0.087).
Patients with low MA were significantly older (mean 64 vs 48 years, P⩽0.001) and had a significantly lower SMA (126.11 vs 156.54 cm2, P⩽0.001), SMI (44.75 vs 52.88 cm2 m−2, P⩽0.001) and FFM (53.02 vs 46.5 kg, P=0.022) compared with those without low MA, as a result patients’ with low MA had a higher prevalence of sarcopenia (45.8% vs 12.5%, P=0.004). The mean HU in those with and without low MA was 30.3 vs 44.4 HU, respectively (P⩽0.001). Patients with sarcopenia or low MA and those without were otherwise similar in terms of clinical features. No significant association was observed between the prevalence of sarcopenia or low MA and the extent of metastatic disease.
Patients with a BMI ⩾25 kg m−2, as expected weighed more (90.3 vs 65.8 kg, P⩽0.001) and had a significantly higher SMI (54.2 vs 45.01 cm2 m−2, P⩽0.001) compared with those with a BMI <25 kg m−2; however, these patients had a significantly lower MA (37.45 HU vs 44.64 HU, P=0.001). Patients were otherwise similar.
The incidence of high-grade (grades III–IV) AEs and irAEs
The frequencies of ipilimumab-associated AEs and irAEs are shown in Table 3. In our study, 60 (71%) patients experienced an AE and 47 (56%) experienced an irAE. Many AE reported were of grades I–II; however, 35 (42%) patients experienced a high-grade AE, with 25 (30%) patients experiencing a high-grade irAE.
Sarcopenia, low MA and treatment tolerability
No significant difference was observed in the prevalence of any grade I–II AEs or grade I–II irAEs in patients with sarcopenia or low MA and in those without (results not shown).
Patients with sarcopenia experienced more high-grade AEs compared with their non-sarcopenic counterparts (65% vs 34%, P=0.030, univariate OR=3.54, 95% CI: 1.23–10.17, P=0.019) and appeared to be more susceptible to a high-grade irAE (45% vs 25%); however, this did not reach statistical significance. When considering individual high-grade AEs or irAEs separately, only fatigue was significantly more prevalent in sarcopenic patients (35% vs 9.4%, P=0.011) (Supplementary Table 1). Patients with low MA were more likely to experience a high-grade AE (75% vs 31%, P=0.001, univariate OR=7.46, 95% CI: 2.45–22.66, P⩽0.001), and more likely to experience a high-grade irAE (54% vs 23%, P=0.017, univariate OR=4.49, 95% CI: 1.64–13.5, P=0.004). Patients with low MA had a higher incidence of colitis (16.7% vs 2.1%, P=0.039) (Supplementary Table 1).
On multivariate logistic regression analysis (Table 4) including age, gender, BMI, sarcopenia and low MA, patients with sarcopenia, low MA and a BMI ⩾25 kg m−2 were at a significantly increased risk of experiencing a high-grade AE (OR=5.34, 95% CI: 1.15–24.88, P=0.033; OR=5.23, 95% CI: 1.41–19.30, P=0.013; and OR=4.01, 95% CI: 1.03–15.69, P=0.046, respectively). Patients with low MA were at a significantly increased risk of experiencing a high-grade irAE (OR=3.57, 95% CI: 1.09–11.77, P=0.036).
Sarcopenia, low MA and the incidence of a DLT
Overall, 15 patients (18%) experienced a DLT. Twelve patients (14.3%) discontinued ipilimumab treatment because of a high-grade irAE, with the most common events being skin toxicity (rash) (four patients), diarrhoea (two patients) and colitis (two patients). Six patients experienced a dose delay, while three patients experienced both a dose delay and early cessation of treatment. Patients who experienced a DLT were older (53 years vs 63 years, P=0.017) and had a significantly lower mean MA (41.2 HU vs 33.4 HU, P=0.003). The prevalence of DLT was more common in patients with low MA compared with those without low MA (37.5% or 9 of 24 vs 10.4% or 5 of 48, P=0.011). Sarcopenic patients appeared to be more susceptible to experience a DLT (25.0% or 5 of 20 vs 15.6% or 10 of 64, respectively); however, this did not reach statistical significance.
Changes in body composition
Longitudinal changes in body composition during four cycles of ipilimumab were examined in patients where both pre- and post-treatment CT images were available (n=59).
Significant decreases were observed across all body composition parameters (see Table 5). The mean decrease in SMA was 3.3% (s.d. 5.84%) (95% CI: −4.48 to −1.79%, P⩽0.001) per 100 days. The prevalence of sarcopenia increased from 17% (or 10 of 59) at baseline to 32% (or 19 of 59) by the second CT scan in this subgroup of patients.
Effect of change on survival
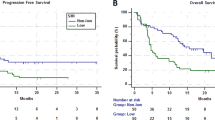
Patients with a muscle loss of ⩾7.5%/100 days (quartile four; highest muscle loss) had significantly lower overall survival compared with those with a muscle loss <7.5%/100 days (quartiles 1–3; minor muscle loss/stable or gain). The median survival was 9.5 months (95% CI: 1.5–17.6 months) for those with the highest muscle loss (⩾7.5%) vs 21.8 months (95% CI: 14.3–29.4 months) in those with a muscle loss <7.5%/100 days (log rank; P=0.029; univariate Cox regression, HR: 2.14, 95% CI: 1.06–4.28, P=0.033) (Figure 1). On multivariate analysis, muscle loss of ⩾7.5% remained independently associated with shorter survival when adjusted for gender, age (<65 vs >65 years) and stage of disease (M1c vs M1a or M1b) as potential confounders (HR: 2.1, 95% CI: 1.02–4.56, P=0.046). Neither sarcopenia nor low MA at baseline was associated with shorter survival.
Discussion
To our knowledge, this is the first study to examine the relationship between body composition and ipilimumab toxicity in patients with metastatic melanoma. Our findings point to sarcopenia and low MA as potential sources of variation in toxicity to ipilimumab.
Overall, 24% of our cohort was sarcopenic before commencing treatment and 33% of these patients had a low MA. We are not aware of any other reports that describe the prevalence of sarcopenia or low MA in patients with metastatic melanoma. The prevalence of sarcopenia in this cohort is considerably lower than previously reported for other advanced stage cancers (Tan et al, 2009; Huillard et al, 2013).
In our study, 56% of patients experienced an irAE of any grade, while 30% of patients experienced a high-grade irAE (grades III and IV). The incidence of toxicity in this cohort is similar to those reported previously (Bertrand et al, 2015; Horvat et al, 2015). A recent meta-analysis published in 2015, which included 81 articles and a total of 1265 patients from 22 clinical trials (Bertrand et al 2015), reported the overall incidence of all-grade irAE to be 61% (95% CI: 56–66%) and the incidence of high-grade irAEs to be 17% (95% CI: 10–23%) for patients receiving an ipilimumab dose of 3 mg kg−1 (Bertrand et al, 2015). Although the incidence of high-grade irAEs in our study (30%) is higher than reported in the meta-analysis (17%), it is not uncommon and is similar with that reported by Horvat et al (2015), where the incidence of high-grade irAE was reported to be 31% in 298 melanoma patients treated with 3 mg kg−1 of ipilimumab (Horvat et al, 2015). The prevalence of toxicity within our study and that of Horvat et al (2015) may be more representative of patients treated in clinical practice and less representative of prior clinical trial participants.
We report that patients with sarcopenia were at an increased risk of experiencing a high-grade AE compared with their non-sarcopenic counterparts (OR=5.34, 95% CI: 1.15–24.88, P=0.033). Several other studies reported associations between sarcopenia and increased chemotherapy toxicity in a variety of cancers including breast (Prado et al, 2009), colon (Prado et al, 2007), oesophageal (Anandavadivelan et al, 2015), thyroid (Massicotte et al, 2013) and renal cancer (Huillard et al, 2013; Cushen et al, 2014). The majority of therapies studied included anticancer drugs based on body surface area (e.g., capecitabine (Prado et al, 2009) and 5-flurouracil (Prado et al, 2007; Barret et al, 2014; Ali et al, 2016), which has been shown to correlate weakly with LBM (r2=0.37) (Prado et al, 2008). Additionally, increased toxicity has been observed in patients with sarcopenia/low LBM treated with flat-dosed targeted therapies such as sunitinib (Huillard et al, 2013; Cushen et al, 2014) and vandetanib (Massicotte et al, 2013).
More recently, reduced MA is increasingly being linked to poorer clinical outcomes in malignant populations. Sabel et al (2011) reported low (psoas) MA to be associated with disease-free (P=0.04) and distant disease-free survival (P=0.0002) in stage III melanoma patients (Sabel et al, 2011). Martin et al (2013) supported these findings and reported low MA was predictive of poorer survival in 1473 lung and GI cancer patients (HR: 1.36, 95% CI: 1.2–1.6) (Martin et al, 2013). In this study, patients with low MA were at an increased risk and more frequently experienced high-grade AEs (OR=5.23, 95% CI: 1.41–19.30, P=0.013; 75% vs 31%, P=0.001) and high-grade irAE (OR=3.57, 95% CI: 1.09–11.77, P=0.036; 54% vs 23%, P=0.017) compared with those without low MA. More importantly, these patients were more susceptible to experience a DLT, either a dose delay or early cessation of treatment as a result of significant toxicity (37.5% vs 10.4%, P=0.011). However, the explanations for these observations are unclear. In contrast to these findings, Rollins et al (2015) found no association between low MA and the incidence of toxicity or ability to complete palliative chemotherapy in pancreatic cancer patients (Rollins et al, 2015) and Blauwhoff-Buskermolen et al (2016) similarly reported no association with baseline sarcopenia or low MA and treatment modifications (DLT) in metastatic colorectal cancer patients (Blauwhoff-Buskermolen et al, 2016). There are few studies examining this relationship. We reported that patients with a BMI >25 kg m−2 were at an increased risk of experiencing a high-grade AE (OR=4.01, 95% CI: 1.03–15.69, P=0.046); however, it is important to note that patients with BMI >25 kg m−2 had a lower MA compared with patients with a normal BMI (P=0.001), and the increased risk of toxicity is most likely attributed to the low MA.
Several explanatory mechanisms of increased toxicity in those with sarcopenia or low lean body mass have been hypothesised (Antoun et al, 2013a). One potential explanation is pharmacokinetic parameter changes induced by malnutrition or obesity, which could result in alterations in the distribution, metabolism and clearance of anticancer drugs, increasing the risk of toxic events. Alternatively, there is a growing body of literature to suggest that patients with sarcopenia are generally more susceptible to acute medical events such as infections (Lieffers et al, 2012), postoperative complications (Peng et al, 2011; van Vugt et al, 2015) and poorer prognosis (Martin et al, 2013; Montano-Loza et al, 2012). Another plausible explanation for increased toxicity in patients with sarcopenia and low MA may be attributed to systemic inflammation, which is known to underlie both conditions. Rollins et al (2015) reported that patients with low MA had significantly greater levels of systemic inflammation (white cell count, neutrophil–lymphocyte ratio and C-reactive protein) than in those without low MA (Rollins et al, 2015). Similarly an association between sarcopenia, low MA and the host inflammatory response was observed in a large cohort (n=763) of patients with operable colorectal cancer (Malietzis et al, 2016).
Treatment toxicity may be as a result of systemic inflammation. It has been reported that patients with a higher inflammatory response had an increased risk of severe hematological toxicity (neutropenic fever or severe thrombocytopenia) following chemotherapy (Alexandre et al, 2003). Similarly, patients with inflammation and lymphopenia at baseline were more susceptible to febrile neutropenia following treatment with docetaxel (Alexandre et al, 2007). Consistent with the expected immune-stimulating effect of ipilimumab, increases in absolute lymphocyte count during treatment have frequently been observed in melanoma patients (Postow et al, 2013). Ipilimumab may augment existing systemic inflammation in patients, resulting in the increased incidence of high-grade AEs in those with sarcopenia and high-grade AEs, irAEs and DLTs in those with low MA.
In our study, patients treated with ipilimumab lost on average 3.32±5.84% of skeletal muscle per 100 days, which is comparable to 3.1±12%/100 days reported in advanced pancreatic cancer patients (Tan et al, 2009), but is at a lower rate compared with ovarian cancer patients undergoing neoadjuvant chemotherapy (5.2±9.8%/100 days) (Rutten et al, 2016). Other studies have reported significant reductions in skeletal muscle in a variety of cancer types (Antoun et al, 2010; Miyamoto et al, 2015; Rollins et al, 2015), but failed to report these as % change/100 days, which makes it difficult to compare with our cohort.
Patients with metastatic melanoma with the highest amount of muscle loss (⩾7.5% highest quartile) were at a significantly increased risk of mortality on multivariate analysis (HR: 2.1, 95% CI: 1.02–4.55; P=0.046). Our results are in line with those reported in colorectal cancer patients, where a loss of muscle >9% SMA over 3 months of chemotherapy was independently associated with reduced survival (HR: 4.47, 95% CI: 2.21–9.05, P<0.001) (Blauwhoff-Buskermolen et al, 2016) and a loss of muscle >2%/100 days was independently associated with significantly reduced survival in ovarian cancer patients (HR: 1.77, 95% CI: 1.018–3.088, P=0.043) (Rutten et al, 2016). In our study, sarcopenia and low MA at baseline were not associated with reduced survival. This is in contrast to some (Prado et al, 2008; Tan et al, 2009; Martin et al, 2013) but not all studies (Stene et al, 2015; Blauwhoff-Buskermolen et al, 2016).
It should be acknowledged that muscle loss is both multifactorial and complex, and significant losses of muscle in this cohort may be attributed to multiple factors. The change in body composition during ipilimumab treatment may simply reflect the evolution of systemic disease; however, response to treatment was not assessed within this patient group. It is often difficult to evaluate the clinical response in patients treated with immunotherapy agents as the patterns of response differ from those with cytotoxic chemotherapy. Radiologically, patients may have a transient worsening of disease, before disease stabilises or regresses and responses in patients can take appreciably longer to become apparent. In line with this, disease regression is frequently observed well after the completion of initial induction period. In addition to catabolic effects caused by the underlying malignancy and advancing disease, muscle loss may be partially explained by the potential systemic inflammation caused by ipilimumab, as well as decreased physical activity and reduced food intake commonly experienced by patients with advanced cancer.
The main limitations of our study are its retrospective nature and relatively small sample size, particularly in the subgroup analysis. Patients were excluded from the analysis if CT scans were not available, which may result in selection bias. The mean time between imaging and initiation of treatment was 39 days (s.d. 31 days), indicating a few outliers outside the ideal target window of 30 days. Owing to the exploratory nature of this study, multiple statistical tests were performed, increasing the risk of committing type 1 errors. These results should be regarded as hypothesis-generating and further prospective studies are needed to validate these results in larger cohort of metastatic melanoma patients as well as in other cancer types and treatments.
However, despite the limitations, our results highlight for the first time the potential use of body composition assessment to identify patients at increased risk of experiencing severe toxicity to immunotherapy, that is, ipilimumab. Additionally, this study reports for the first time the changes in body composition experienced by patients while undergoing treatment with ipilimumab, and the adverse effect this has on survival. Potential treatments to increase muscle mass and their effectiveness to improve clinical outcomes warrants further investigation. With the advent of novel immune checkpoint inhibitors showing significant activity in many solid and liquid tumours, for example, pembrolizumab and nivolumab in melanoma, lung cancer, colon cancer, ovarian cancer, bladder cancer, glioblastima, head and neck cancers, and Hodgkin lymphoma (American Society of Clinical Oncology Education Book 2016), in conjunction with the lack of validated clinical and molecular biomarkers that predict response, outcome and toxicity, the importance of body composition defined by cross-sectional imaging criteria should not be underestimated.
Change history
31 January 2017
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Alexandre J, Gross-Goupil M, Falissard B, Nguyen ML, Gornet JM, Misset JL, Goldwasser F (2003) Evaluation of the nutritional and inflammatory status in cancer patients for the risk assessment of severe haematological toxicity following chemotherapy. Ann Oncol 14 (1): 36–41.
Alexandre J, Rey E, Girre V, Grabar S, Tran A, Montheil V, Rabillon F, Dieras V, Jullien V, Hérait P, Pons G, Treluyer JM, Goldwasser F (2007) Relationship between cytochrome 3 A activity, inflammatory status and the risk of docetaxel-induced febrile neutropenia: a prospective study. Ann Oncol 18 (1): 168–172.
Ali R, Baracos VE, Sawyer MB, Bianchi L, Roberts S, Assenat E, Mollevi C, Senesse P (2016) Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med 5 (4): 607–616.
Anandavadivelan P, Brismar TB, Nilsson M, Johar AM, Martin L (2015) Sarcopenic obesity: a probable risk factor for dose limiting toxicity during neo-adjuvant chemotherapy in oesophageal cancer patients. Clin Nutr 35: 724–730.
Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE (2010) Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J Clin Oncol 28 (6): 1054–1060.
Antoun S, Borget I, Lanoy E (2013a) Impact of sarcopenia on the prognosis and treatment toxicities in patients diagnosed with cancer. Curr Opin Support Palliat care 7 (4): 383–389.
Antoun S, Lanoy E, Iacovelli R, Albiges-Sauvin L, Loriot Y, Merad-Taoufik M, Fizazi K, di Palma M, Baracos VE, Escudier B (2013b) Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 119 (18): 3377–3384.
Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, Latko E, Taieb J (2014) Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer 66 (4): 583–589.
Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T (2015) Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 13: 211.
Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, den Braver NR, Berkhof J, Langius JA, Verheul HM (2016) Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 34 (12): 1339–1344.
Cushen SJ, Power DG, Teo MY, Maceneaney P, Maher MM, McDermott R, O'Sullivan K, Ryan AM (2014) Body composition by computed tomography as a predictor of toxicity in patients with renal cell carcinoma treated with sunitinib. Am J Clin Oncol e-pub ahead of print 21 April 2014.
Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM (2011) Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 16 (1): 5–24.
Heymsfield SB, Wang Z, Baumgartner RN, Ross R (1997) Human body composition: advances in models and methods. Annu Rev Nutr 17: 527–558.
Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363 (8): 711–723.
Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, Carvajal RD, Dickson MA, D'Angelo SP, Woo KM, Panageas KS, Wolchok JD, Chapman PB (2015) Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 33 (28): 3193–3198.
Huillard O, Mir O, Peyromaure M, Tlemsani C, Giroux J, Boudou-Rouquette P, Ropert S, Delongchamps NB, Zerbib M, Goldwasser F (2013) Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br J Cancer 108 (5): 1034–1041.
Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE (2012) Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 107 (6): 931–936.
Malietzis G, Johns N, Al-Hassi HO, Knight SC, Kennedy RH, Fearon KC, Aziz O, Jenkins JT (2016) Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg 263 (2): 320–325.
Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31 (12): 1539–1547.
Massicotte MH, Borget I, Broutin S, Baracos VE, Leboulleux S, Baudin E, Paci A, Deroussent A, Schlumberger M, Antoun S (2013) Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: results from a placebo-controlled study. J Clin Endocrinol Metab 98 (6): 2401–2408.
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 85 (1): 115–122.
Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Watanabe M, Baba H (2015) Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLoS One 10 (6): e0129742.
Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB (2012) Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 10 (2): 166–173.
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33 (5): 997–1006.
Peng PD, van Vledder MG, Tsai S, de Jong MC, Makary M, Ng J, Edil BH, Wolfgang CL, Schulick RD, Choti MA, Kamel I, Pawlik TM (2011) Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 13 (7): 439–446.
Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P (2004) Nutritional assessment: lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr 79 (4): 613–618.
Postow MA, Chasalow SD, Yuan J, Kuk D, Panageas KS, Cheng M, Shahabi V, Berman DM, Wolchok JD (2013) Pharmacodynamic effect of ipilimumab on absolute lymphocyte count (ALC) and association with overall survival in patients with advanced melanoma. J Clin Oncol 31 (Suppl): abstract 9052.
Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, Butts CA, Scarfe AG, Sawyer MB (2007) Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 13 (11): 3264–3268.
Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15 (8): 2920–2926.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9 (7): 629–635.
Prado CM, Lima IS, Baracos VE, Bies RR, McCargar LJ, Reiman T, Mackey JR, Kuzma M, Damaraju VL, Sawyer MB (2011) An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol 67 (1): 93–101.
Robert C, Ghiringhelli F (2009) What is the role of cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma? Oncologist 14 (8): 848–861.
Rollins KE, Tewari N, Ackner A, Awwad A, Madhusudan S, Macdonald IA, Fearon KC, Lobo DN (2015) The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr.
Rutten IJ, van Dijk DP, Kruitwagen RF, Beets-Tan RG, Olde Damink SW, van Gorp T (2016) Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle 7: 458–466.
Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S (2011) Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol 18 (13): 3579–3585.
Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 97 (6): 2333–2338.
Stene GB, Helbostad JL, Amundsen T, Sørhaug S, Hjelde H, Kaasa S, Grønberg BH (2015) Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol 54 (3): 340–348.
Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC (2009) Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 15 (22): 6973–6979.
van Vugt JL, Braam HJ, van Oudheusden TR, Vestering A, Bollen TL, Wiezer MJ, de Hingh IH, van Ramshorst B, Boerma D (2015) Skeletal muscle depletion is associated with severe postoperative complications in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 22 (11): 3625–3631.
Weber JS, Kähler KC, Hauschild A (2012) Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 30 (21): 2691–2697.
Acknowledgements
We would like to acknowledge the support of the Health Research Board Clinical Research Facility, Cork (CRF-C). This publication has emanated from research conducted with the financial support of Science Foundation Ireland (SFI) under Grant Number SFI/12/RC/2273.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Daly, L., Power, D., O'Reilly, Á. et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer 116, 310–317 (2017). https://doi.org/10.1038/bjc.2016.431
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.431
Keywords
This article is cited by
-
Sarcopenia is associated with leukopenia in urothelial carcinoma patients who receive tislelizumab combined with gemcitabine and cisplatin therapy
International Journal of Clinical Oncology (2024)
-
Nutrition care is an integral part of patient-centred medical care: a European consensus
Medical Oncology (2023)
-
Immune-related toxicity and soluble profile in patients affected by solid tumors: a network approach
Cancer Immunology, Immunotherapy (2023)
-
Association of computed tomography-based body composition with survival in metastatic renal cancer patient received immunotherapy: a multicenter, retrospective study
European Radiology (2022)
-
The impact of nutritional risk factors and sarcopenia on survival in patients treated with pelvic exenteration for recurrent gynaecological malignancy: a retrospective cohort study
Archives of Gynecology and Obstetrics (2022)