Abstract
Background:
Neuroendocrine tumours (NET) overexpress somatostatin receptors (SSTR) that can be targeted for therapy. Somatostatin receptor expression is routinely measured by molecular imaging but the resolution is insufficient to define heterogeneity. We hypothesised that SSTR expression could be measured on circulating tumour cells (CTCs) and used to investigate heterogeneity of expression and track changes during therapy.
Methods:
MCF-7 cells were transfected with SSTR2 or 5 and spiked into donor blood for analysis by CellSearch. Optimum anti-SSTR antibody concentration and exposure time were determined, and flow cytometry was used to evaluate assay sensitivity. For clinical evaluation, blood was analysed by CellSearch, and SSTR2/5 immunohistochemistry was performed on matched tissue samples.
Results:
Flow cytometry confirmed CellSearch was sensitive and that detection of SSTR was unaffected by the presence of somatostatin analogue up to a concentration of 100 ng ml−l. Thirty-one NET patients were recruited: grade; G1 (29%), G2 (45%), G3 (13%), primary site; midgut (58%), pancreatic (39%). Overall, 87% had SSTR-positive tumours according to somatostatin receptor scintigraphy or 68-Ga-DOTATE PET/CT. Circulating tumour cells were detected in 21 out of 31 patients (68%), of which 33% had evidence of heterogeneous expression of either SSTR2 (n=5) or SSTR5 (n=2).
Conclusions:
Somatostatin receptors 2 and 5 are detectable on CTCs from NET patients and may be a useful biomarker for evaluating SSTR-targeted therapies and this is being prospectively evaluated in the Phase IV CALMNET trial (NCT02075606).
Similar content being viewed by others
Main
Gastroenteropancreatic (GEP) neuroendocrine tumours (NETs) represent a heterogeneous disease entity with diverse biological and clinical features. They are characterised histologically by high expression of somatostatin receptors (Yao et al, 2008), of which five different subtypes have been identified. The most commonly expressed is SSTR2, followed by SSTR1, SSTR5 and SSTR3, whereas SSTR4 is the least expressed subtype (de Herder et al, 2003; Reubi, 2011). This unique expression profile has been successfully exploited for both diagnostic and therapeutic applications through the use of somatostatin analogues (SA), which bind with high affinity to SSTR2 and SSTR5 (Fazio et al, 2010). Somatostatin analogues are commonly used to control symptoms arising from hormone hypersecretion in functional NETs, and recent randomised trials have also demonstrated an anti-proliferative effect resulting in delayed tumour progression (Rinke et al, 2009; Caplin et al, 2014). Somatostatin receptor expression has also been investigated as a potential prognostic factor and SSTR2a but not SSTR5 expression has been shown to be an independent positive prognostic factor for survival in pancreatic NET although prospective validation remains outstanding (Mehta et al, 2015).
In routine clinical practice, SSTR expression is evaluated by imaging using scintigraphy or positron emission tomography (PET) but the resolution of these modalities is insufficient to define intra-tumoural heterogeneity of SSTR expression, nor is imaging the optimal method to track changes in expression that may arise during therapy. We hypothesised that SSTR expression could be measured on circulating tumour cells (CTCs) and provide insights into the heterogeneity of expression as well as a means of tracking expression over time and during therapy. Using the CellSearch system, we have previously demonstrated that CTCs are detectable in patients with NET and that their presence is an adverse prognostic factor (Khan et al, 2011a, 2013b). In addition, we have shown that early changes in CTC numbers predict survival in response to therapy (Khan et al, 2015). Here we describe the development of a CTC-based assay for detecting SSTR expression and its application in a cohort of GEP NET patients who have correlative imaging and histological data regarding SSTR expression.
Materials and methods
Cell lines
In order to develop the assay, we generated EpCAM-positive cells that expressed either SSTR2 or 5. Human breast cancer MCF-7 cells were transiently transfected with a mammalian expression vector carrying full-length human SSTR2 or SSTR5 using GeneJuice reagent (Merck KGaA, Darmstadt, Germany) according to the transfection reagent kit protocol under the following optimised conditions; MCF-7 cells were grown to 80% confluence in MEM medium with 2 M glutamine, 1% non-essential amino acids and 10% foetal bovine serum (FBS) in 24-well tissue culture plates at 37 °C and humidified with 5% CO2. Plasmid pcDNA6.2/hSSTR2 (provided by Ipsen, Slough, UK) was mixed with the GeneJuice transfection reagent at a ratio of 1.5 μl transfection reagent to 0.5 μg DNA and transfection performed in complete medium for 48 h prior to trypsinising and freezing at −80 °C in FBS with 10% DMSO. Transfection efficiency was assessed by growing cells on glass coverslips and fixing with 4% paraformaldehyde for 10 min. Cells were subsequently permeabilised in phosphate buffered saline (PBS) with 0.5% Tween for 15 min and blocked in PBS with 5% bovine serum albumin (blocking solution) for 30 min. Coverslips were then incubated with 36 μg ml−1 anti-SSTR2 Antibody (UMB1, Abcam, Cambridge, UK; ab134152) or 14.8 μg ml−1 anti-SSTR5 Antibody (UMB4, Abcam; ab109495) in blocking solution for 1 h. The primary antibody was washed off with PBS and the coverslips incubated with Alexa Fluor 488 Goat Anti-Rabbit IgG (H+L) Antibody A11008 (Life Technologies, Carlsbad, CA, USA) diluted 1 : 200 in blocking solution for a further hour. The secondary antibody was washed off with PBS and the coverslips mounted on slides using ProLong Gold Antifade Mountant with DAPI (Life Technologies, P-36931). Cells were imaged using the Zeiss Axio M1 microscope to confirm expression of SSTR2 and SSTR5, respectively.
Detection of SSTR expression using CellSearch
Cells were spiked into healthy donor blood and analysed using the CellSearch platform. This semiautomated system enriches for CTCs by EpCAM targeted immunomagnetic selection, following which CTCs are identified by positive immunofluorescent staining for pan-cytokeratin and 4,2-diamidino-2-phenylindole-dihydrochloride (DAPI), and negative staining for the leucocyte marker CD45. A fluorescein isothiocyanate-conjugated antibody can be added to the fourth fluorescence channel to further characterise cells for an additional marker of interest. For this study, anti-SSTR2 antibody (UMB1, Abcam; ab134152) and anti-SSTR5 antibody (UMB4, Abcam; ab109495) were provided as Alexa-488 conjugates by Abcam. Cells were defined as positive for SSTR expression when staining was present in the fourth channel. Test runs were performed for each receptor on the Veridex CellTracks Autoprep System and CellTracks Analyzer II in order to determine optimal antibody concentrations and scan time. Three validation runs were performed using these conditions and spiked healthy donor blood samples.
Flow cytometry
In order to investigate the sensitivity of SSTR2 and SSTR5 detection using the CellSearch platform, expression levels were also quantified by flow cytometry for direct comparison. Transfected MCF-7 cells were prepared as previously described and harvested using trypsin. Approximately 500 cells were spiked into healthy donor blood and analysed by CellSearch as previously described. The remaining cells from the same harvest were prepared for flow cytometry as follows; cells were re-suspended in 1 ml PBS, centrifuged and re-suspended in 0.5 ml paraformaldehyde prior to incubation at room temperature for 8 min. A further centrifugation step was performed before samples were washed in 1 ml PBS and re-suspended in 700 μl PBS for storage at 4 °C. To evaluate SSTR expression, cells were stained in triplicate in 96 well plates using 50 μg ml−1 SSTR2 (UMB1, Abcam; ab134152) or 10 μg ml−1 SSTR5 (UMB4, Abcam; ab109495) Alexa-488-conjugated antibodies and analysed using a BD Fortessa X20 (BD Biosciences, Cambridge, UK). The percentage of single cells positive for SSTR2 or 5 was then calculated and compared with that detected using CellSearch.
To establish the effect of SA on the ability to detect SSTR expression in CTCs, the expression analysis was also performed in the presence of SA. Transfected MCF-7 cells were treated with either 0, 10 or 100 ng ml−1 lanreotide (BIM-23014; provided by Ipsen). Untransfected cells were also treated identically and used as negative control cells. After 16 h overnight incubation, cells were trypsinised and fixed with 4% paraformaldehyde and stored at 4 °C. Cells were stained in triplicate with 50 μg ml−1 SSTR2 or 10 μg ml−1 SSTR5 and analysed by BD Fortessa X20 (BD Biosciences). Between 2000–10 000 events were counted and the median fluorescent intensity (MFI) was recorded and plotted.
Patients
To be eligible for the study patients were required to have histologically confirmed NET of midgut or pancreatic origin or of unknown primary, and metastatic disease measurable by Response Evaluation Criteria in Solid Tumours (RECIST). Data were collected on age, gender, primary site and grade according to the European Neuroendocrine Tumour Society (ENETS) guidelines, and presence of uptake on Gallium-68 Dotatate PET/CT or Indium-111 pentetreotide scintigraphy. This study was approved by the Local Ethics Committee and all participants were required to provide written informed consent.
Immunohistochemistry
Tumours were classified according to primary site and graded according to the ENETS guideline (Rindi et al, 2006). Sections (3 μm) of tumour tissue were deparaffinised in xylene and rehydrated in graded alcohols. Sections were then placed in 0.5% hydrogen peroxidase in methanol for 10 min to block endogenous peroxidase activity. Microwave antigen retrieval was performed for 20 min in citrate buffer. Immunohistochemical staining was carried out using the NovoLink polymer detection system (Novocastra, Leica Microsystems, Milton Keynes, UK). A section from each tissue specimen was incubated with either (i) rabbit anti-SSTR2 antibody (UMB1, Abcam; ab134152) or (ii) rabbit anti-SSTR5 antibody (UMB4, Abcam; ab109495) at a dilution of 1 : 100 for 1 h at room temperature. Samples then underwent post-primary block for 30 min followed by NovoLink polymer for 30 min in a humidity chamber at room temperature. Reaction products were visualised using freshly prepared NovoLink DAB (3,3’-diaminobenzidine tetrahydrochloride) solution for 10 min by adding 50 μl of DAB chromagen to 1 ml of Novolink DAB substrate buffer. Slides were counterstained with Mayer’s haematoxylin and mounted. Normal pancreatic tissue served as internal positive controls. The semi-quantitative analysis of the stained sections was performed by an independent pathologist without any knowledge of pathological data using the DAKO HER2/neu score (Pentheroudakis et al, 2011).
Evaluation of CTCs from NET patients
For each patient, two 7.5 ml blood samples were collected into evacuated CellSave tubes (Janssen Diagnostics, Raritan, NJ, USA) and maintained at room temperature. All samples were processed within 96 h of collection. The CellSearch platform was used for detection and enumeration of CTCs as previously described (Khan et al, 2011b). Analysis of SSTR2 and SSTR5 expression on CTCs was performed using Alexa Fluor 488-conjugated Somatostatin Receptor 2 antibody and Alexa Fluor 488-conjugated Somatostatin Receptor 5 antibody at pre-determined concentrations and exposure times. Cells were defined as positive for SSTR2 or SSTR5 when fourth channel staining was present. All evaluations regarding enumeration of CTCs and expression of SSTR2 and SSTR5 were made by two independent operators without knowledge of patient pathology.
Results
Detection of SSTR expression in spiked cells
Approximately 500 SSTR2- and SSTR5-transfected MCF-7 cells were spiked separately into 7.5 ml healthy donor blood. A range of antibody concentrations (SSTR2: 50 and 100 μg ml−1; SSTR5; 10 μg ml−1) and three different exposure times (0.1, 0.8 and 4.0 s) were used to scan samples using a CellSearch CTC Kit on the CellTracks Autoprep and the Analyzer II (Figure 1A). In order to calculate optimal antibody concentration and exposure time, SSTR2 and 5 positive cells were enumerated and compared with expected values based on the calculated transfection rate for that sample (SSTR2; 16%, SSTR5; 14%) and input number of spiked cells. A scanning exposure time of 0.8 s and antibody concentration of 50 μg ml−1 for SSTR2 and 10 μg ml−1 for SSTR5 was found to be optimal. Three validation runs were subsequently performed using these conditions and healthy donor blood. For each run, four samples using blood from a healthy donor, and a CellSearch CTC control sample were analysed. The four samples included: (i) healthy donor blood alone with anti-SSTR antibody, (ii) healthy donor blood spiked with 500 untransfected MCF-7 cells with anti-SSTR antibody, (iii) healthy donor blood spiked with 500 transfected MCF-7 cells with anti-SSTR antibody and (iv) healthy donor blood spiked with 500 transfected MCF-7 cells analysed without anti-SSTR antibody. For each run the acceptance criteria for receptor expression were met. Of the four sample types, only the samples spiked with transfected MCF-7 cells in the presence of anti-SSTR antibody contained cells staining positively for SSTR2 or 5. The number of positive cells detected for both receptors fell within the expected range of 10–20% based on the observed transfection efficiency for that sample.
CellTracks Analyzer II images. (A) SSTR transfected MCF-7-spiked blood samples. (i) SSTR2-positive tumour cell. (ii) SSTR2-negative tumour cell. (iii) SSTR5-positive tumour cell. (iv) SSTR5-negative tumour cell. (B) Clinical validation in patient samples. (i) SSTR2-positive CTC in patient 21. (ii) SSTR2-negative CTC in patient 21. (iii) SSTR5-positive CTC in patient 7. (iv) SSTR5-negative CTC in patient 7.
Sensitivity of SSTR2 and 5 detection using CellSearch
Using the CellSearch platform, cells were defined as either positive or negative for SSTR expression according to the staining pattern observed in the fourth channel. To investigate whether the CellSearch was sufficiently sensitive to detect a low level of SSTR expression, we compared the results from CellSearch directly with flow cytometric analysis. Two separate transfections were performed for each receptor and despite the transfection efficiency varying between the two transfections, the overall percentage of marker positive cells was comparable across the two different methodologies (Table 1 and Supplementary Figure 1). These experiments confirmed the specificity of the SSTR2 and SSTR5 antibodies and the sensitivity of the CellSearch to detect a wide range of SSTR2 and 5 expression levels.
Effect of SA treatment on detection of SSTR2 and 5 expression on spiked cells
As it is important clinically to be able to assess SSTR expression in patients receiving ongoing treatment, we evaluated the ability of our assay to detect SSTR expression in the presence of background SA. In order to quantify this, we used flow cytometry to measure the MFI in MCF-7 cells transfected with SSTR2 or SSTR5 in the presence of increasing doses of lanreotide. The mean steady-state trough serum lanreotide concentration in patients with GEPNETs range between 5.3 and 8.6 ng ml−1, and we therefore treated our transfected cells with a range of concentrations up to a supratherapeutic dose of 100 ng m−1 (SOMATULINE® DEPOT (lanreotide) INJECTION, 2015). The MFI remained the same for both the SSTR2- and SSTR5-transfected cells when treated with 0, 10 and 100 ng ml−1 of lanreotide (Table 2, Supplementary Figure 2) indicating that detection of SSTR expression is not affected by SA therapy.
Patients for CTC evaluation
Between November 2014 and August 2015, 32 patients with metastatic NET were recruited from the Royal Free Hospital, London. One sample was excluded due to a hardware malfunction during enrichment leading to sample loss. The remaining 31 patients were included in this study and their characteristics are shown in Table 3. Notably, the majority of patients had midgut tumours (58%), were grade 1 or 2 (74%) and had positive uptake on somatostatin receptor imaging (87%). Overall, 61% were receiving therapy with SA at the time of recruitment.
SSTR detection in CTC from metastatic NET patients
Peripheral blood samples from 31 patients with metastatic NET were analysed by the CellSearch system adapted for SSTR2 and SSTR5 detection using the optimised conditions previously described. As shown in Table 4, CTCs were detected in 21 (68%) patients (midgut; n=14, pancreatic; n=6; unknown primary; n=1, range; 1–636). Seven patients out of 21 (33%) showed a subpopulation of CTCs expressing either SSTR2 (n=5) or SSTR5 (n=2) (Table 4 and Figure 1B). These patients had G1 or G2 tumours and no patients with G3 tumours had SSTR(+)CTCs. In those patients with SSTR(+) CTCs, the fraction of SSTR2(+) or SSTR5(+) CTCs varied from 10 to 100% and 50 to 100%, respectively, indicating intra-patient heterogeneity of SSTR expression. No patients had evidence of both SSTR2(+)and SSTR5(+)expression. Five out of the seven patients with SSTR2(+)or SSTR5(+) CTC subpopulations were on active treatment with SA at time of sample collection, confirming previous flow cytometry studies indicating that SA therapy does not interfere with detection of SSTR2 or SSTR5 using this assay.
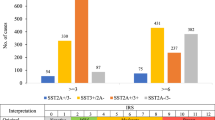
Immunohistochemical analysis of SSTR2 and 5 expression
Of the 21 patients with detectable CTCs, 11 had blocks of formalin-fixed and paraffin-embedded tissue (FFPE) available for further immunohistochemistry (IHC). Staining for SSTR2 and 5 was predominantly membranous, although some cytoplasmic staining was seen in occasional cases (Figure 2). Moderate to strong staining was seen in 10 out of 11 cases for SSTR2 and only 3 out of 11 cases for SSTR5. A further three cases showed weak staining for SSTR2 (Table 4). The concordance between the IHC and CTC staining for SSTR 2 and 5 was variable; patients 9, 19 and 24 were positive for SSTR2 on tissue and CTCs and patient 21 was positive for SSTR 5 on both. However, patients 7, 9,10, 25 and 27 had SSTR5 expression on tissue but not CTCs, whereas patients 5, 10, 20, 21, 25, 27 and 29 had SSTR2 expression on tissue but none in CTCs. Conversely, patient 5 had SSTR5 expression on CTCs but not tissue, and patient 7 had SSTR2 expression on CTCs but not tissue.
Discussion
In clinical practice, expression of SSTR2 and 5 in NET patients is measured by scintigraphy or PET imaging. However, there can be heterogeneity in SSTR2 and 5 expression with metastatic sites exhibiting different expression profiles when compared with the primary tumour (Nasir et al, 2006; Hofman and Hicks, 2012; Kaemmerer et al, 2015). Imaging lacks the resolution necessary to visualise differences in expression profiles on a single-cell basis and is not practical to track changes in expression over time or with therapy. By contrast, CTCs provide a potential method to sample the tumour tissue directly for expression relevant therapeutic targets at a single-cell level and at multiple time points. Other groups have previously demonstrated that targets such as HER2, ER/PR and PD-L1 can be evaluated in CTCs and this may inform treatment selection and stratification (Pestrin et al, 2009; Munzone et al, 2010; Punnoose et al, 2010; Mazel et al, 2015). To our knowledge, SSTR expression has not been evaluated previously in CTCs and our study provides the first such analysis.
In keeping with previously published data (Khan et al, 2013a), CTCs were isolated in 68% of metastatic NET patients overall, with a higher proportion of midgut patients having detectable CTCs (78%) compared with pancreatic patients (50%). In those with detectable CTCs, we show that SSTR2 and 5 expression can be found in a subpopulation of CTCs in 33% of patients and that SSTR2 expression is more commonly observed than SSTR5, consistent with the existing literature for immunohistochemistry (de Herder et al, 2003; Reubi, 2011; Kaemmerer et al, 2012). However, it is noteworthy that 12 of the 14 patients with no detectable SSTR(+) CTC subpopulations had tumours that were positive for SSTR expression as determined by functional imaging. This difference is unlikely to be explained by sampling bias as large numbers of CTCs were found in four discordant cases ranging from 34 to 636. There was also some degree of discordance between immunohistochemistry and CTC expression despite the fact that the same antibody clones were used for both. For SSTR2, there was positive expression in seven cases by IHC but not in CTCs and for one case, there was expression in CTCs but not in tissue. For SSTR5, there was positive expression in five cases by IHC, which were negative in CTCs and one case where the reverse was true. There are a number of explanations that may account for these discrepancies. First, the expression analysis for either CTC or IHC may not be sufficiently sensitive or specific. For CTCs, the data from both the assay development and the fluorescence activated cell sorting (FACS) analysis suggest that this was not the case. The CellSearch and FACS analysis demonstrated marker positive cells only in transfected cell populations, and the FACS analysis showed a range of SSTR expression levels in a proportion of cells that was equivalent to that detected by CellSearch. In addition, the fact that some cells were clearly positive within a population of negative cells suggests that these were truly negative. Collectively, these findings suggest that the CellSearch assay for SSTR2 and 5 is both sensitive and specific. Regarding IHC, other groups have demonstrated that tissue immunoreactive scores for SSTR2 and SSTR5 correlate with both 68-Ga DOTATOC PET/CT and somatostatin receptor scintigraphy, and also with RT-qPCR for SSTR2 quantification (Volante et al, 2007; Miederer et al, 2009; Kaemmerer et al, 2011; Kaemmerer et al, 2015). Consistent with this, all patients in our study with positive SSTR2 or 5 staining by IHC had corresponding positive SSTR imaging suggesting that the IHC is reliable. A second possible reason for discrepancy might be that both imaging and histology are historical rather than contemporaneous with respect to CTC analysis, and the reduced SSTR expression seen in CTCs may arise from tumour evolution. Neuroendocrine tumour patients often have an indolent disease course resulting in an increased time between initial tumour sampling and CTC collection. It has been reported that SSTR status can change over the course of disease progression (Krenning et al, 2005; Gabriel et al, 2007) and in keeping with this Kaemmerer et al, 2015 have shown that SSTR2 expression as assessed by IHC is significantly higher in pancreatic NET primary tumours than at metastatic sites. This could lead to a positive result by IHC despite a negative test in CTCs; the two patients (patients 5 and 7) with complete discordance between CTC and archived tissue expression profiles in our study had IHC performed on archived samples that were 31 and 85 months old, respectively, and both originated from primary tumours. However, this is unlikely to completely explain the discordance seen, particularly given that serial imaging in most patients demonstrate the persistence of SSTR over years. A third and most likely explanation is that there is heterogeneity of expression within tumours and between CTCs. Our results clearly demonstrate heterogeneity in both tissue and CTCs, where small subpopulations show clear expression of SSTR while the majority of cells are negative. However, as mentioned, there are four cases with large numbers of CTCs that are all negative, whereas the corresponding imaging is positive. These observations may reflect the biology and/or plasticity of metastasising cells in which SSTR-negative subpopulations are more likely to metastasise or whose phenotype changes during translocation in the blood. It is interesting to note that the two high-grade tumours with many CTCs were both negative for SSTR expression.
Other groups have also reported heterogeneity or discordance between biomarker expression on CTCs and archived tissue. For example, several groups have investigated HER2 expression on CTCs and archived tissue in breast cancer patients and found discordance rates varying between 11 and 33% (Pestrin et al, 2009; Flores et al, 2010; Munzone et al, 2010; Punnoose et al, 2010). Concordance rates also appear to be lower when comparing CTC biomarker expression to primary tumour samples as compared with metastatic disease sites.
Our findings, particularly the discordance between CTC and tissue expression, might have profound implications for therapy and partly explain the escape from disease control seen in patients treated with SA or peptide receptor radionuclide therapy (PRRT). In addition, they provide intriguing insights into the phenotypic characteristics of metastasising cells, which clearly needs further evaluation. A key question, therefore, is the relevance of SSTR expression on CTCs for patient management, and this is currently being tested in the ongoing Phase IV CALM-NET study (NCT02075606). In this multicentre prospective trial, our assay is being used to investigate the relationship between SSTR2 and 5 expression on CTCs and progression-free survival in patients with functioning midgut NET receiving treatment with Lanreotide Autogel. Circulating tumour cells are being enumerated at multiple time points during therapy and SSTR status evaluated. It is anticipated that this longitudinal study will provide novel insights into the role of CTCs as pharmacodynamics markers in this disease.
Change history
06 December 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P CLARINET Investigators (2014) Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371: 224–233.
de Herder WW, Hofland LJ, van der Lely AJ, SWJ Lamberts (2003) Somatostatin receptors in gastroentero-pancreatic neuroendocrine tumours. Endocr Relat Cancer 10: 451–458.
Fazio N, Cinieri S, Lorizzo K, Squadroni M, Orlando L, Spada F, Maiello E, Bodei L, Paganelli G, Delle Fave G, De Braud F (2010) Biological targeted therapies in patients with advanced enteropancreatic neuroendocrine carcinomas. Cancer Treat Rev 36 (Suppl 3): S87–S94.
Flores LM, Kindelberger DW, Ligon AH, Capelletti M, Fiorentino M, Loda M, Cibas ES, Jänne PA, Krop IE (2010) Improving the yield of circulating tumour cells facilitates molecular characterisation and recognition of discordant HER2 amplification in breast cancer. Br J Cancer 102: 1495–1502.
Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, Kovacs P, Guggenberg Von E, Bale R, Virgolini IJ (2007) 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med 48: 508–518.
Hofman MS, Hicks RJ (2012) Changing paradigms with molecular imaging of neuroendocrine tumors. Discov Med 14: 71–81.
Kaemmerer D, Peter L, Lupp A, Schulz S, Sänger J, Baum RP, Prasad V, Hommann M (2012) Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int J Clin Exp Pathol 5: 187–194.
Kaemmerer D, Peter L, Lupp A, Schulz S, Sänger J, Prasad V, Kulkarni H, Haugvik S-P, Hommann M, Baum RP (2011) Molecular imaging with 68Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 38: 1659–1668.
Kaemmerer D, Wirtz RM, Fischer EK, Hommann M, Sänger J, Prasad V, Specht E, Baum RP, Schulz S, Lupp A (2015) Analysis of somatostatin receptor 2A immunohistochemistry, RT-qPCR, and in vivo PET/CT data in patients with pancreatic neuroendocrine neoplasm. Pancreas 44: 648–654.
Khan MS, Kirkwood A, Tsigani T, Garcia-Hernandez J, Hartley JA, Caplin ME, Meyer T (2013a) Circulating tumor cells as prognostic markers in neuroendocrine tumors. J Clin Oncol 31: 365–372.
Khan MS, Kirkwood AA, Tsigani T, Lowe H, Goldstein R, Hartley JA, Caplin ME, Meyer T (2015) Early changes in circulating tumor cells are associated with response and survival following treatment of metastatic neuroendocrine neoplasms. Clin Cancer Res 22: 79–85, clincanres.1008.2015.
Khan MS, Luong TV, Watkins J, Toumpanakis C, Caplin ME, Meyer T (2013b) A comparison of Ki-67 and mitotic count as prognostic markers for metastatic pancreatic and midgut neuroendocrine neoplasms. Br J Cancer 108: 1838–1845.
Khan MS, Tsigani T, Rashid M, Rabouhans JS, Yu D, Luong TV, Caplin M, Meyer T (2011a) Circulating tumor cells and EpCAM expression in neuroendocrine tumors. Clin Cancer Res 17: 337–345.
Khan MS, Tsigani T, Rashid M, Rabouhans JS, Yu D, Luong TV, Caplin M, Meyer T (2011b) Circulating tumor cells and EpCAM expression in neuroendocrine tumors. Clin Cancer Res 17: 337–345.
Krenning EP, Valkema R, Kwekkeboom DJ, de Herder WW, van Eijck CHJ, de Jong M, Pauwels S, Reubi JC (2005) Molecular imaging as in vivo molecular pathology for gastroenteropancreatic neuroendocrine tumors: implications for follow-up after therapy. J Nucl Med 46 (Suppl 1): 76S–82S.
Mazel M, Jacot W, Pantel K, Bartkowiak K, Topart D, Cayrefourcq L, Rossille D, Maudelonde T, Fest T, Alix-Panabières C (2015) Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol 9: 1773–1782.
Mehta S, de Reuver PR, Gill P, Andrici J, D'Urso L, Mittal A, Pavlakis N, Clarke S, Samra JS, Gill AJ (2015) Somatostatin receptor SSTR-2a expression is a stronger predictor for survival than Ki-67 in pancreatic neuroendocrine tumors. Medicine (Baltimore) 94: e1281.
Miederer M, Seidl S, Buck A, Scheidhauer K, Wester H-J, Schwaiger M, Perren A (2009) Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging 36: 48–52.
Munzone E, Nolé F, Goldhirsch A, Botteri E, Esposito A, Zorzino L, Curigliano G, Minchella I, Adamoli L, Cassatella MC, Casadio C, Sandri M-T (2010) Changes of HER2 status in circulating tumor cells compared with the primary tumor during treatment for advanced breast cancer. Clin Breast Cancer 10: 392–397.
Nasir A, Stridsberg M, Strosberg J, Su P-H, Livingston S, Malik HA, Kelley ST, Centeno BA, Coppola D, Malafa ME, Yeatman TJ, Kvols LK (2006) Somatostatin receptor profiling in hepatic metastases from small intestinal and pancreatic neuroendocrine neoplasms: immunohistochemical approach with potential clinical utility. Cancer Control 13: 52–60.
Pentheroudakis G, Batistatou A, Kalogeras KT, Kronenwett R, Wirtz RM, Bournakis E, Eleftheraki AG, Pectasides D, Bobos M, Papaspirou I, Kamina S, Gogas H, Koutras AK, Pavlidis N, Fountzilas G (2011) Prognostic utility of β-tubulin isotype III and correlations with other molecular and clinicopathological variables in patients with early breast cancer: a translational Hellenic Cooperative Oncology Group (HeCOG) study. Breast Cancer Res Treat 127: 179–193.
Pestrin M, Bessi S, Galardi F, Truglia M, Biggeri A, Biagioni C, Cappadona S, Biganzoli L, Giannini A, Di Leo A (2009) Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res Treat 118: 523–530.
Punnoose EA, Atwal SK, Spoerke JM, Savage H, Pandita A, Yeh R-F, Pirzkall A, Fine BM, Amler LC, Chen DS, Lackner MR (2010) Molecular biomarker analyses using circulating tumor cells. PLoS One 5: e12517.
Reubi JC (2011) Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev 24: 389–427.
Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, Körner M, Lopes JM, McNicol A-M, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B all other Frascati Consensus Conference participants European Neuroendocrine Tumor Society (ENETS) (2006) TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 449: 395–401.
Rinke A, Müller H-H, Schade-Brittinger C, Klose K-J, Barth P, Wied M, Mayer C, Aminossadati B, Pape U-F, Bläker M, Harder J, Arnold C, Gress T, Arnold R PROMID Study Group (2009) Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 27: 4656–4663.
SOMATULINE® DEPOT (lanreotide) INJECTION (2015) Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022074s011lbl.pdf.
Volante M, Brizzi MP, Faggiano A, La Rosa S, Rapa I, Ferrero A, Mansueto G, Righi L, Garancini S, Capella C, De Rosa G, Dogliotti L, Colao A, Papotti M (2007) Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol 20: 1172–1182.
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey J-N, Rashid A, Evans DB (2008) One hundred years after ‘carcinoid’: epidemiology of and prognostic factors for neuroendocrine tumors in 35 825 cases in the United States. J Clin Oncol 26: 3063–3072.
Acknowledgements
The funding was provided by the University College London (UCL) Experimental Cancer Medicine Centre Grant No. C12125/A15576, the UCL Hospitals NIHR Biomedical Research Centre and Ipsen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
TM has received research funding for this work from Ipsen, MEC has received grant funding and consultancy fees from Ipsen.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Childs, A., Vesely, C., Ensell, L. et al. Expression of somatostatin receptors 2 and 5 in circulating tumour cells from patients with neuroendocrine tumours. Br J Cancer 115, 1540–1547 (2016). https://doi.org/10.1038/bjc.2016.377
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.377
Keywords
This article is cited by
-
The Quest for Circulating Biomarkers in Neuroendocrine Neoplasms: a Clinical Perspective
Current Treatment Options in Oncology (2023)
-
Circulating tumour cells and their association with bone metastases in patients with neuroendocrine tumours
British Journal of Cancer (2019)
-
Clinical applications of (epi)genetics in gastroenteropancreatic neuroendocrine neoplasms: Moving towards liquid biopsies
Reviews in Endocrine and Metabolic Disorders (2019)
-
Importance of Immunohistochemical Detection of Somatostatin Receptors
Pathology & Oncology Research (2019)
-
Role of biomarker tests for diagnosis of neuroendocrine tumours
Nature Reviews Endocrinology (2018)