Abstract
Background:
The antiproliferative activity of octreotide LAR in neuroendocrine tumours (NETs) has been demonstrated by small retrospective studies and confirmed by a prospective phase III trial (PROMID). However, there are limited data about the duration and predictors of response. The aim of our retrospective study was to determine the time to radiological progression (TTRP) of disease and the factors that were associated with better response.
Methods:
A total of 254 treatment naïve patients with advanced NETs and positive somatostatin receptor scintigraphy were included. Mean follow-up period was 42 months.
Results:
The location of primary was in the small bowel in 204, pancreas in 22, lungs in 14, rectum in 7 and unknown in 7 patients. Most tumours were well-differentiated, G1 (58%) and G2 (23%). The majority of patients commenced octreotide LAR due to functional symptoms (57%), radiological progression (10%) or in the presence of asymptomatic and stable disease on the basis of data from the PROMID trial (18.5%). Partial response occurred in 5%. For all patients, the median TTRP was 37 months (95% confidence interval, CI: 32–52 months). There was a statistically significant shorter TTRP in patients with pancreatic tumours, liver metastases and intermediate grade tumours. Extremely raised (>10 times the upper limit of normal) baseline chromogranin A levels were associated with an unfavourable outcome. In contrast, male sex, carcinoid heart disease and initiation of treatment in the presence of stable disease were predictive of a better response. Age, extra-hepatic metastases, presence of mesenteric desmoplasia, previous resection and functional status of the primary tumour did not affect response.
Conclusions:
The duration of the antiproliferative effect of octreotide LAR seems to be longer than previously reported. This study has identified several predictors of response in a large cohort of patients with NETs on somatostatin analogue therapy.
Similar content being viewed by others
Main
Neuroendocrine tumours (NETs) are rare neoplasms with an incidence between 2 and 9.3 per 100 000 persons per year (Ramage et al, 2012). They represent a heterogeneous group of neoplasms, arising from neuroendocrine cells derived from the diffuse endocrine system. They may be associated with a specific syndrome due to hormone hypersecretion, which differentiates ‘functional’ from ‘non-functional’ tumours.
Somatostatin analogues (SSA) are commonly used to treat symptoms associated with hormone hypersecretion and have a favourable safety profile. The antiproliferative activity of SSA in NETs has been recently confirmed in two randomised placebo-controlled trials (Rinke et al, 2009; Toumpanakis and Caplin, 2013; Caplin et al, 2014). The PROMID trial was a placebo-controlled, double-blind, randomised study assessing the effect of octreotide LAR on the control of tumour growth in patients with metastatic, well-differentiated midgut NETs. The study demonstrated that octreotide LAR significantly lengthened the time to tumour progression compared with placebo (median time to tumour progression 14.3 months vs 6 months, respectively; Rinke et al, 2009). More recently, another randomised, double-blind, placebo-controlled trial evaluated the effect of lanreotide in patients with metastatic, well- or moderately differentiated, non-functional enteropancreatic NETs (the CLARINET trial). This study showed that lanreotide was associated with a significantly prolonged progression-free survival compared with placebo (median progression-free survival not reached vs 18 months, respectively; Caplin et al, 2014).
However, there are limited data regarding the duration of response and factors predictive of tumour control in patients with NETs on SSA therapy. The aims of the present study were to determine the mean time to radiological progression (TTRP) of disease and factors affecting radiological response to SSA therapy in a large cohort of patients with NETs treated with octreotide LAR.
Materials and methods
Patients
A total of 254 patients with NETs treated with octreotide LAR (as monotherapy) in our centre from 2001 to 2014, were included in this retrospective study. All patients had confirmed histopathological diagnosis. All patients were treatment-naïve, but primary tumour resection before somatostatin analogue therapy was not an exclusion criterion. In addition, all patients had a positive somatostatin receptor scintigraphy before commencement of octreotide LAR.
Somatostatin analogue therapy
All patients (who were somatostatin naïve) had an initial test dose of subcutaneous (s.c.) octreotide (50 μg). If no immediate adverse effect was noted, patients either had an initial 2-week course of s.c. octreotide (100/200 μg tds) and then were switched over to octreotide LAR or patients were given monthly octreotide LAR after no reaction to a single test dose of s.c. octreotide. The initial dose of octreotide LAR was 20 mg/28 days in 198 patients and 30 mg/28 days in 56 patients.
Study design
A retrospective review of patient electronic records was performed. Data collected included patient demographics (age, sex), data on the characteristics of treatment with octreotide LAR (dose, indication), tumour characteristics (location of primary, functional status, grade, presence of carcinoid heart disease and mesenteric desmoplasia, presence of liver or extra-hepatic metastases, extent of liver involvement, resection of primary and TTRP), and biochemical data (baseline serum chromogranin A (CgA)).
NET tumours were retrospectively classified and graded according to WHO 2010 (Bosman et al, 2010). Tumours were graded into three levels based on tumour cell proliferation: G1: mitotic count<2 per 10 high-power fields and/or Ki67⩽2%; G2: mitotic count 2–20 per 10 high-power fields and/or Ki67 3–20%; G3: mitotic count>20 per 10 high-power fields and/or Ki67⩾20% (Rindi et al, 2007).
Extent of liver involvement (0: absence of liver metastases, 1: volume of liver metastases affecting <25% of hepatic parenchyma, 2: volume of liver metastases affecting 25–50% of hepatic parenchyma, 3: volume of liver metastases affecting >50% of hepatic parenchyma) was evaluated by one experienced radiologist (L.G.). The presence of mesenteric desmoplasia was assessed by computed tomography imaging. However, assessment of the extent or degree of desmoplastic reaction was not performed due to the absence of established classification systems.
The aims of this retrospective study were to demonstrate the mean TTRP, as well as identify factors affecting the antiproliferative activity of octreotide LAR in a large cohort of patients with NETs.
Radiological assessment was based on cross-sectional imaging in 4–6 month intervals, using the revised RECIST (version 1.1) criteria (Eisenhauer et al, 2009). Many variables were assessed as potential predictive factors of response to treatment with octreotide LAR: age, sex, treatment indication, location of primary tumour, extent of liver involvement, extra-hepatic metastases (bone, peritoneum, mesentery), presence of carcinoid heart disease and mesenteric desmoplasia, tumour grade, functional status (functional or non-functional), resection of primary tumour and baseline CgA level.
Statistical analysis
Non-parametric Kaplan–Meier techniques were used to evaluate times to radiological progression, including the median and associated 95% CI, in strata defined by the various explanatory variables. A semi-parametric Cox regression model was used to perform multivariate analysis on a sample of 152 patients with complete information on all covariates of interest (Collett, 2003). By including fewer covariates, additional multivariate models were fitted to larger patient samples to test the robustness of the findings to potential sample bias.
Results
Patient characteristics
A total of 254 patients were included in this retrospective study. The patients’ mean age was 60.5±12.8 years. The male to female ratio was 1.1 : 1. The location of the primary tumour was in the small bowel in 204 (80%), pancreas in 22 (8.5%), lung in 14 (5.5%), rectum in 7 (2.5%) and unknown in 7 patients (2.5%). The histological grade was G1 in 148 (58%), G2 in 59 (23%), G3 in 1 (0.5%) and not available in 47 patients (18.5%). The study population included 183 patients with functional tumours (72%) (of which 97% were serotonin secreting, 1% glucagonomas, 1.5% gastrinomas and 0.5% insulinomas), 67 with non-functional tumours (26%), whereas data on functional status were not available in 4 patients (2%). The primary tumour had been resected before commencement of octreotide LAR in 117 (46%) patients. Indications for commencement of octreotide LAR included functional symptoms in 57%, radiological progression in 10%, a combination of functional symptoms and radiological progression in 3%, whereas 18.5% of patients were asymptomatic with stable disease and started treatment on the basis of data from the PROMID trial (CLARINET trial data were not available during the period covered in this retrospective cohort study). The duration of stable disease before initiation of SSA therapy in this group of patients was at least 3 months. A total of 8% of patients were commenced on octreotide LAR due to a combination of functional symptoms and stable disease (on the basis of data from the PROMID trial) and the indication was not clearly recorded in 3.5% of cases.
A summary of patient baseline characteristics is provided in Table 1.
Partial (objective) response, TTRP and univariate analysis of predictive factors of tumour control during treatment with octreotide LAR
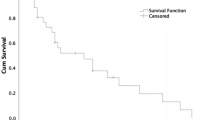
Partial (objective) response was noted in 13 patients (5%). For all patients the median and mean TTRP were 37 months (95% CI: 32–52 months) and 51 months (95% CI: 45–57 months), respectively (Figure 1).
Kaplan–Meier estimate of the survival function for the time to tumour progression for all 254 patients. The crosses indicate right-censored data whereby patients’ tumours were stable at the last time of contact before being lost to follow-up. The median time to tumour progression is 37 months (95% CI: 32–52 months), given by the time at which the Kaplan–Meier estimate of the survival function is 0.5. The mean time to tumour progression is 51 months (95% CI: 45–57 months), calculated using a restricted upper time limit of 120 months.
The univariate analysis demonstrated that liver and skeletal metastases, G2 tumours and baseline serum CgA levels more than 10 times the upper limit of normal were predictive factors of a shorter TTRP, whereas male sex was associated with a statistically significantly longer TTRP.
Pancreatic and rectal primary tumours demonstrated a trend towards shorter TTRP and tumours associated with carcinoid heart disease demonstrated a trend towards longer TTRP, but both associations did not reach the level of statistical significance.
In contrast, age, treatment indication, peritoneal or mesenteric metastases, functional status, presence of mesenteric desmoplasia and resection of primary tumour were not found to be predictive factors affecting response to treatment.
The results of the univariate analysis are shown in Table 2.
Multivariate analysis of predictive factors of tumour control during treatment with octreotide LAR
The multivariate analysis demonstrated that G2 tumours, liver metastases and pancreatic primary tumours were predictors of a shorter TTRP. In contrast, male sex and presence of carcinoid heart disease were predictors of a longer TTRP. In addition, initiation of treatment in asymptomatic patients with stable disease was predictive of a better response to octreotide LAR.
Age, presence of skeletal, peritoneal and mesenteric metastases, functional status, tumour resection and presence of mesenteric desmoplasia were not found to be predictive of tumour response to therapy.
The results of the multivariate analysis are shown in Table 3.
Two additional multivariate analysis models including fewer co-variates were fitted to larger patient samples with complete data, in order to assess the validity of these findings given the potential risk of sampling bias (Supplementary Tables 1 and 2).
TTRP stratified by factors predictive of response to treatment with octreotide LAR
The following factors were identified by multivariate analysis as predictive of a favourable or unfavourable response to treatment with octreotide LAR and the TTRP stratified by each of these factors was calculated separately:
Tumour grade
The median TTRP was 45 months (95% CI: 33–72 months) for patients with G1 tumours and 21 months (95% CI: 15–37 months) for patients with G2 tumours (Figure 2).
Liver involvement
The median TTRP was 105 months (95% CI: 36, NA) for patients without liver metastases, 37 months (95% CI: 26–79 months) for patients with volume of liver metastases <25%, 23 months (95% CI: 13, NA) for patients with volume of liver metastases 25–50% and 33 months (95% CI: 20, NA) for patients with volume of liver metastases >50% (Figure 2).
Location of primary tumour
The median TTRP was 20 months (95% CI: 12–79 months) for patients with a pancreatic primary tumour, 43 months (95% CI: 32–64 months) for patients with a small bowel primary, 4 months (95% CI: 11, NA) for patients with a lung primary and 8 months (95% CI: 4, NA) for those with a rectal primary (Figure 2).
Treatment indication
The median TTRP was 53 months (95% CI: 28, NA) for asymptomatic patients with stable disease. The median TTRP was 32 months (95% CI: 27–51 months) for patients whose indication was functional symptoms and 50 months (95% CI: 35, NA) for patients whose indication was radiological progression. The median TTRP was 43 months (95% CI: 21, NA) for patients with a combination of functional symptoms and radiological progression and 20 months (95% CI: 19, NA) for patients, whose indications were both functional symptoms and stable disease (initiated on SSA therapy on the basis of evidence from the PROMID trial; Figure 2).
Carcinoid heart disease
The median TTRP was 36 months (95% CI: 30–51 months) for patients without carcinoid heart disease and 72 months (95% CI: 33, NA) for patients with carcinoid heart disease (Figure 2).
A subsequent analysis of electronic records of patients with carcinoid heart disease revealed that the majority of patients had mild carcinoid heart disease with NYHA class I (54%) or II (37.5%). Most of these patients (92%) also had N-terminal pro b-type natriuretic peptide (NT pro-BNP) values of <400 pg ml−1, which has been proposed as a cut-off value predictive of a diagnosis of chronic heart failure (Al-Mohammad et al, 2010).
Sex
The median TTRP was 32 months (95% CI: 23–45 months) for female and 49 months (95% CI: 36–96 months) for male patients (Figure 2).
Discussion
Partial (objective) response and TTRP
The present study demonstrated that in a large cohort of patients with NETs on octreotide LAR the partial (objective) response rate was 5%. This is in keeping with the results of other prospective and retrospective studies, which have shown partial (objective) response rates of 0–10% (Saltz et al, 1993; Arnold et al, 1996; di Bartolomeo et al, 1996; Eriksson et al, 1997; Wymenga et al, 1999; Ducreux et al, 2000; Aparicio et al, 2001; Shojamanesh et al, 2002; Faiss et al, 2003; Rinke et al, 2009; Toumpanakis et al, 2009; Martín-Richard et al, 2013; Palazzo et al, 2013; Ramundo et al, 2014; Supplementary Table 3).
However, in our study the TTRP was 37 months, which is longer than that reported in most other studies (Arnold et al, 1996; Shojamanesh et al, 2002; Butturini et al, 2006; Rinke et al, 2009; Toumpanakis et al, 2009; Palazzo et al, 2013; Martín-Richard et al, 2013; Caplin et al, 2014; Supplementary Table 3). It is possible that differences in the baseline patient and tumour characteristics among various studies may account for this variation in TTRP.
Predictive factors of tumour response
There is only a small number of studies that have identified predictors of tumour response to SSA therapy and these are summarised in Supplementary Table 4.
Our study is the largest retrospective study in the literature aiming to determine predictive factors of radiological response to SSA therapy. Its large sample size has allowed the analysis of multiple variables as potential predictive factors. Such analysis was not feasible in previous studies due to the small study samples. Some of our study findings are in keeping with those of previously published studies, although some differences are also present.
In our study, the multivariate analysis revealed that G2 tumours were less likely to respond to octreotide LAR. Similarly, a small prospective study demonstrated that lower proliferation index (Ki67) predicted superior tumour growth control, although this association did not quite reach the level of statistical significance, perhaps due to the small sample size (Martín-Richard et al, 2013). Another small prospective study which included advanced, well-differentiated, non-functional pancreatic NETs concluded that tumour progression correlated with a Ki67⩾5%, weight loss and absence of abdominal pain (Butturini et al, 2006).
Our study also showed that liver metastases are predictive of a poor response to treatment. Palazzo et al (2013) also found that Ki67<5%, hepatic tumour load<25% and pre-treatment stability were associated with disease stability on lanreotide therapy. The PROMID trial also suggested that the antiproliferative activity of octreotide LAR was more pronounced in patients with a low (⩽10%) hepatic tumour load and a resected primary tumour (Rinke et al, 2009). A previous retrospective study performed in our centre showed that in a subgroup of patients with G1 tumours, bilobar liver metastases at the time of commencement of treatment and plasma CgA levels>1000 pmol l−1 were predictive of radiological progression (Toumpanakis et al, 2009). Interestingly, our univariate analysis also showed that CgA levels>10 times the upper limit of normal were predictive of a poor response, although this could not be confirmed in the multivariate analysis due to missing data in a large number of subjects.
In addition, our multivariate analysis showed that pancreatic tumours were less likely to respond to treatment. A previous prospective study also demonstrated that patients with midgut NETs showed a statistically significant prolonged length of time to tumour progression compared with foregut tumours (most of which were pancreatic), although the study population included not only patients treated with lanreotide, but also patients treated with interferon or a combination of both drugs (Faiss et al, 2003).
Moreover, our results demonstrated that asymptomatic patients with stable disease were more likely to respond to treatment. Similarly, other studies have shown that rapidly progressive tumours are less likely to respond to treatment compared with stable or slowly progressive tumours before initiation of SSA (Aparicio et al, 2001; Shojamanesh et al, 2002; Palazzo et al, 2013).
The present study also revealed that male sex and carcinoid heart disease were favourable predictive factors of tumour response. These variables were statistically significant across all multivariate analysis models. This was an unexpected finding, as carcinoid heart disease is associated with poor prognosis. However, an analysis of the electronic case notes of patients with carcinoid heart disease revealed that the vast majority of cases had only mild disease and it is possible that they were perhaps followed-up and treated more aggressively, which resulted in a more favourable outcome.
Finally, primary tumour resection has been identified in other studies as predictive of a better response to SSA therapy (Panzuto et al, 2006). In our study, this factor was not found to be significant in any of the multivariate analysis models.
Although many of our study findings are in keeping with those of other studies, some differences are also present. These may be due to differences in predictive factors assessed, heterogeneity of study populations, differences in sample sizes and use of different SSAs amongst various studies. In practical terms, however, decisions regarding initiation of SSA therapy still need to be made on a case-by-case basis, although these may be influenced by the presence of associated predictive factors.
Limitations and conclusions
There are several limitations to this study. Because of retrospective data collection the results are potentially subject to interpretation bias and bias because of missing data. In addition, this is a single-centre cohort study in a tertiary and quaternary referral centre which attracts many referrals outside its geographical catchment area. Therefore, the profile of our patients and clinical practices may not be typical of regional centres and the extrapolation of our findings to other populations can be questioned. It is difficult to quantify the degree of potential bias, but the fact that many of our results are in keeping with those of previous studies suggests that this may not be a major area of concern. Furthermore, in our study the vast majority of patients had small bowel primary tumours (n=204) followed by pancreatic (n=22), lung (n=14) and rectal primaries (n=7). Therefore, our results may be more reliable for patients with small bowel and pancreatic NETs and less robust for other NETs. Finally, the majority of patients with functional tumours were those with carcinoid syndrome as a result of a small bowel primary NET. Only a minority of patients had functional pancreatic tumours, such as insulinoma or glucagonoma. As a result, our findings on the impact of functional status on the antiproliferative activity of SSA therapy mainly reflect the presence or not of carcinoid syndrome.
In conclusion, the current study suggests that the duration of the anti-proliferative effect of octreotide LAR is longer than previously reported. Male sex, carcinoid heart disease and possibly disease stability at the time of commencement of SSA therapy are predictive of a better response, although the vast majority of patients had minor cardiac disturbance and might have been treated and followed-up more aggressively resulting in a more positive outcome. Age, extra-hepatic metastases (other than perhaps skeletal), mesenteric desmoplasia, tumour functional status and primary tumour resection do not appear to have any effect on treatment. In contrast, liver (and perhaps skeletal) metastases, intermediate grade tumours, pancreatic primary tumours and possibly extremely raised CgA levels may be associated with an unfavourable response.
Change history
22 November 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Al-Mohammad A, Mant J, Laramee P (2010) Diagnosis and management of adults with chronic heart failure: summary of updated NICE guidance. BMJ 341: c4130.
Aparicio T, Ducreux M, Baudin E, Sabourin JC, De Baere T, Mitry E, Schlumberger M, Rougier P (2001) Antitumour activity of somatostatin analogues in progressive metastatic neuroendocrine tumours. Eur J Cancer 37 (8): 1014–1019.
Arnold R, Trautmann ME, Creutzfeldt W, Benning R, Benning M, Neuhaus C, Jürgensen R, Stein K, Schäfer H, Bruns C, Dennler HJ (1996) Somatostatin analogue octreotide and inhibition of tumour growth in metastatic endocrine gastroenteropancreatic tumours. Gut 38 (3): 430–438.
Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO Classification of Tumours of the Digestive System 4th edn. Lyon: IARC.
Butturini G, Bettini R, Missiaglia E, Mantovani W, Dalai I, Capelli P, Ferdeghini M, Pederzoli P, Scarpa A, Falconi M (2006) Predictive factors of efficacy of the somatostatin analogue octreotide as first line therapy for advanced pancreatic endocrine carcinoma. Endocr Relat Cancer 13 (4): 1213–1221.
Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P CLARINET Investigators (2014) Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371 (3): 224–233.
Collett D (2003) Modelling Survival Data in Medical Research. Chapman & Hall: London.
di Bartolomeo M, Bajetta E, Buzzoni R, Mariani L, Carnaghi C, Somma L, Zilembo N, di Leo A (1996) Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors. A study by the Italian Trials in Medical Oncology Group. Cancer 77 (2): 402–408.
Ducreux M, Ruszniewski P, Chayvialle JA, Blumberg J, Cloarec D, Michel H, Raymond JM, Dupas JL, Gouerou H, Jian R, Genestin E, Hammel P, Rougier P (2000) The antitumoral effect of the long-acting somatostatin analog lanreotide in neuroendocrine tumors. Am J Gastroenterol 95 (11): 3276–3281.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45 (2): 228–247.
Eriksson B, Renstrup J, Imam H, Oberg K (1997) High-dose treatment with lanreotide of patients with advanced neuroendocrine gastrointestinal tumors: clinical and biological effects. Ann Oncol 8 (10): 1041–1044.
Faiss S, Pape UF, Böhmig M, Dörffel Y, Mansmann U, Golder W, Riecken EO, Wiedenmann B (2003) Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferonalfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors—the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol 21 (14): 2689–2696.
Martín-Richard M, Massutí B, Pineda E, Alonso V, Marmol M, Castellano D, Fonseca E, Galán A, Llanos M, Sala MA, Pericay C, Rivera F, Sastre J, Segura A, Quindós M, Maisonobe P TTD (Tumores del Tracto Digestivo) Study Group (2013) Antiproliferative effects of lanreotide autogel in patients with progressive, well-differentiated neuroendocrine tumours: a Spanish, multicentre, open-label, single arm phase II study. BMC Cancer 13: 427.
Palazzo M, Lombard-Bohas C, Cadiot G, Matysiak-Budnik T, Rebours V, Vullierme MP, Couvelard A, Hentic O, Ruszniewski P (2013) Ki67 proliferation index, hepatic tumor load, and pretreatment tumor growth predict the antitumoral efficacy of lanreotide in patients with malignant digestive neuroendocrine tumors. Eur J Gastroenterol Hepatol 25 (2): 232–238.
Panzuto F, Di Fonzo M, Iannicelli E, Sciuto R, Maini CL, Capurso G, Milione M, Cattaruzza MS, Falconi M, David V, Ziparo V, Pederzoli P, Bordi C, Delle Fave G (2006) Long-term clinical outcome of somatostatin analogues for treatment of progressive, metastatic, well-differentiated entero-pancreatic endocrine carcinoma. Ann Oncol 17 (3): 461–466.
Ramage J, Ahmed A, Ardill J, Bax N, Breen D, Caplin M, Corrie P, Davar J, Davies A, Lewington V, Meyer T, Newell-Price J, Poston G, Reed N, Rockall A, Steward W, Thakker RV, Toubanakis C, Valle J, Verbeke C, Grossman AB UK and Ireland Neuroendocrine Tumour Society (2012) Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut 61 (1): 6–32.
Ramundo V, Del Prete M, Marotta V, Marciello F, Camera L, Napolitano V, De Luca L, Circelli L, Colantuoni V, Di Sarno A, Carratù AC, de Luca di Roseto C, Colao A, Faggiano A Multidisciplinary Group for Neuroendocrine Tumors of Naples (2014) Impact of long-acting octreotide in patients with early-stage MEN1-related duodeno-pancreatic neuroendocrine tumours. Clin Endocrinol (Oxf) 80 (6): 850–855.
Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes J, McNicol A, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B (2007) TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch 451: 757–762.
Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, Harder J, Arnold C, Gress T, Arnold R PROMID Study Group (2009) Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 27 (28): 4656–4663.
Saltz L, Trochanowski B, Buckley M, Heffernan B, Niedzwiecki D, Tao Y, Kelsen D (1993) Octreotide as an antineoplastic agent in the treatment of functional and nonfunctional neuroendocrine tumors. Cancer 72 (1): 244–248.
Shojamanesh H, Gibril F, Louie A, Ojeaburu JV, Bashir S, Abou-Saif A, Jensen RT (2002) Prospective study of the antitumor efficacy of long-term octreotide treatment in patients with progressive metastatic gastrinoma. Cancer 94 (2): 331–343.
Toumpanakis C, Caplin M (2013) Update on the role of somatostatin analogs for the treatment of patients with gastroenteropancreatic neuroendocrine tumors. Semin Oncol 40 (1): 56–68.
Toumpanakis C, Garland J, Marelli L, Srirajaskanthan R, Soh J, Davies P, Buscombe J, Caplin ME (2009) Long-term results of patients with malignant carcinoid syndrome receiving octreotide LAR. Aliment Pharmacol Ther 30 (7): 733–740.
Wymenga AN, Eriksson B, Salmela PI, Jacobsen MB, Van Cutsem EJ, Fiasse RH, Välimäki MJ, Renstrup J, de Vries EG, Oberg KE (1999) Efficacy and safety of prolonged-release lanreotide in patients with gastrointestinal neuroendocrine tumors and hormone-related symptoms. J Clin Oncol 17 (4): 1111.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
MC and CT are on the advisory boards. They received research funding and hononoraria for lectures from NOVARTIS and IPSEN. The remaining authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Laskaratos, FM., Walker, M., Naik, K. et al. Predictive factors of antiproliferative activity of octreotide LAR as first-line therapy for advanced neuroendocrine tumours. Br J Cancer 115, 1321–1327 (2016). https://doi.org/10.1038/bjc.2016.349
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.349