Abstract
Background:
Little is known about modifiable behaviours that may be associated with epithelial ovarian cancer (EOC) survival. We conducted a pooled analysis of 12 studies from the Ovarian Cancer Association Consortium to investigate the association between pre-diagnostic physical inactivity and mortality.
Methods:
Participants included 6806 women with a primary diagnosis of invasive EOC. In accordance with the Physical Activity Guidelines for Americans, women reporting no regular, weekly recreational physical activity were classified as inactive. We utilised Cox proportional hazard models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) representing the associations of inactivity with mortality censored at 5 years.
Results:
In multivariate analysis, inactive women had significantly higher mortality risks, with (HR=1.34, 95% CI: 1.18–1.52) and without (HR=1.22, 95% CI: 1.12–1.33) further adjustment for residual disease, respectively.
Conclusion:
In this large pooled analysis, lack of recreational physical activity was associated with increased mortality among women with invasive EOC.
Similar content being viewed by others
Main
Epithelial ovarian cancer (EOC) is the most deadly gynaecological cancer in developed nations (Torre et al, 2015). Five-year survival is approximately 46% in the United States and Europe (SEER, 2014; UK CR, 2015). Among women with invasive EOC, over 60% are diagnosed with advanced-stage disease, with considerably worse 5-year survival, ranging from 3 to 27% in the United States and the United Kingdom (SEER, 2014; UK CR, 2015). While recent reports of improved long-term survival have been promising (Akeson et al, 2009; Wright et al, 2015), most women diagnosed with advanced-stage EOC will die from their disease, generally within 5 years of diagnosis.
The most commonly cited prognostic factors associated with invasive EOC survival are unmodifiable, and include disease stage and grade at diagnosis, histology, and the extent of residual disease remaining after tumour resection (Winter et al, 2007; Cress et al, 2015; Wright et al, 2015). While little is known about modifiable behaviours that may be associated with EOC prognosis, the lack of recreational physical activity, defined by the Physical Activity Guidelines for Americans (PAGA) as engaging in no regular, weekly, moderate-, or vigorous-intensity exercise during leisure time (USDHHS, 2008), is a potentially modifiable behavioural target for improving prognosis (Sanchis-Gomar et al, 2015; Li et al, 2016).
Worldwide, over 31% of adults are physically inactive, but inactivity increases with age and is higher among women than men (Hallal et al, 2012). As an exposure variable, inactivity can be assessed with less misclassification than incremental categories of physical activity (Bull et al, 2004; Celis-Morales et al, 2012). Inactivity may also reflect physiological pathways that affect carcinogenesis independently from pathways associated with obesity or physical activity and skeletal muscle contraction (Fiuza-Luces et al, 2013; Byers, 2014; Hildebrand et al, 2015; Sanchis-Gomar et al, 2015). Few studies have systematically evaluated the association between physical inactivity and ovarian cancer prognosis. Thus, we chose to examine the association of physical inactivity with subsequent mortality in women diagnosed with invasive EOC.
Materials and Methods
We conducted a pooled analysis utilising individual-level data from 12 studies in the Ovarian Cancer Association Consortium (OCAC) (Berchuck et al, 2008). Study protocols were approved by the respective institutional review boards, and participants provided written informed consent. The study population included 6806 women aged 18 years and older, with histologically confirmed primary diagnoses of invasive EOC, fallopian tube cancer, or primary peritoneal cancer.
Analysis variables
Mortality was assessed with time-to-event analyses censored at 5 years. Thus, women were followed from the date of diagnosis to the earliest of date of death, date of last follow-up, or 5 years after the date of diagnosis. Available covariates included a comprehensive set of epidemiological and clinical variables from the OCAC core data set, which was collated, reviewed, cleaned, and harmonised for use in OCAC pooled analyses.
Physical activity was assessed using self- or interviewer-administered questionnaires. Questionnaire format for assessing physical activity habits varied between studies, but all questionnaires allowed for the identification of inactive women as defined by the PAGA. Women reporting no regular moderate- to vigorous-intensity recreational physical activities were categorised as inactive, our exposure of interest. Questionnaires from nine studies (AUS, CON, DOV, HAW, MAL, NEC, NJO, USC, and HOP; Table 1) yielded data reflecting pre-diagnostic activity spanning the course of adulthood, while questionnaires from three studies (JPN, MAY, and MAC; Table 1) yielded data reflecting activity at enrollment. To reduce the likelihood of reverse causation as an explanation for observed associations, we conducted sensitivity analyses excluding the three studies assessing inactivity at enrollment.
Statistical methods
Multivariable Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) representing the association between physical inactivity and mortality risk. We examined mortality overall and according to subgroups by tumour histology, tumour stage, menopausal status, and body mass index (BMI) classification. We pre-specified age at diagnosis, tumour stage, and histology as important adjustment variables; additional confounders were identified utilising the 10% change-in-estimate guide (Maldonado and Greenland, 1993). While the extent of residual disease after surgical resection is a well-established prognostic factor for invasive EOC, these data were only available in a subset of participants (N=2473). Therefore, we estimated the association between inactivity and mortality through two multivariable models, with and without adjustment for residual disease. Finally, between-study heterogeneity for the association between inactivity and mortality was assessed by means of Q-statistics (P<0.05) and I-squared statistics (<50%) (Higgins et al, 2003).
Results
During the follow-up period, 2935 participants (43.1%) died. All but one study (MAC) included herein were case–control studies and nine studies originated in the United States (Table 1). Participants were mostly white, post-menopausal women with advanced-stage high-grade serous EOC. Collectively, 24.5% of participants self-reported inactivity before diagnosis (Supplementary Table S1).
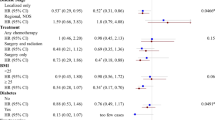
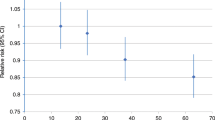
For the association of inactivity with mortality, we observed no significant heterogeneity between studies (Q-statistic P=0.21; I-squared=23.7%), nor evidence of a site-by-inactivity interaction (P=0.12). Therefore, we estimated pooled multivariable HRs and 95% CIs utilising a combined data set. Inactive women had significantly greater risk of mortality (HR=1.22, 95% CI: 1.12–1.33) (Table 2); the association remained significant with adjustment for residual disease (HR=1.34, 95% CI: 1.18–1.52; Table 3). Further control for smoking and BMI did not affect the significant increased risk of mortality among inactive women with (HR=1.35, 95% CI: 1.16–1.56) or without (HR=1.16, 95% CI: 1.05–1.27) adjustment for residual disease.
In subgroup analyses by histology, inactive women with high-grade serous tumours had significantly higher mortality risks in models without adjustment for residual disease (HR=1.21, 95% CI: 1.11–1.33; Table 2). In models adjusted for residual disease, inactive women with high-grade serous and clear cell tumours had significantly greater mortality than their active counterparts: HR=1.36 (95% CI: 1.17–1.58) and HR=1.73 (95% CI: 1.06–2.84), respectively (Table 3). Because we were insufficiently powered to detect associations among the more infrequent histological subtypes, we also limited histology classifications to serous vs non-serous disease. Here we observed consistent evidence of the association between inactivity and mortality for both tumour types, both with and without adjustment for residual disease (Supplementary Table S2).
In sensitivity analyses intended to reduce possible reverse causation bias by exclusion of the three studies that assessed inactivity only at enrollment, associations between inactivity and mortality remained significant and were similar in magnitude to the associations observed in our primary analysis: HR=1.28 (95% CI: 1.09–1.49) and HR=1.19 (95% CI: 1.09–1.30) in models with and without adjustment for residual disease, respectively. In additional analyses excluding women who had died within 1 year of diagnosis, the associations between inactivity and mortality remained significant and of similar magnitude to those in primary analyses in models both with (HR=1.27, 95% CI: 1.10–1.47) and without (HR=1.18, 95% CI: 1.08–1.29) adjustment for residual disease. Finally, we observed no evidence of effect modification of the association between inactivity and mortality by tumour stage (Supplementary Table S3), menopausal status (Supplementary Table S4), or overweight/obesity status (Supplementary Table S5).
Discussion
The current analyses of pooled individual-level data from OCAC suggests that self-reported, habitual recreational physical inactivity is an independent predictor of mortality among women diagnosed with invasive EOC. The observed associations between inactivity and mortality were consistently seen in sensitivity analyses designed to reduce potential biases and were robust to adjustment for relevant confounders and well-established prognostic factors. Importantly, physical inactivity remained an independent predictor of mortality even among participants diagnosed with advanced disease. If the association with pre-diagnostic activity also applies to physical activity after ovarian cancer diagnosis, it is possible that targeted intervention to reduce inactivity, adjuvant to medical management, could improve survival in women with EOC. This association needs confirmation by a large randomised trial.
Several biological mechanisms have been proposed to account for an association between physical inactivity and cancer development, including increased adiposity, increased circulating sex hormones, chronic inflammation, impaired immune surveillance, impaired insulin regulation, and dysregulated adipokines (McTiernan, 2008). These same mechanisms could explain some of the observed mortality risks associated with physical inactivity in cancer survivors (Li et al, 2016). Further, obesity and physical inactivity may affect carcinogenesis through independent pathways (Byers, 2014; Hildebrand et al, 2015; Sanchis-Gomar et al, 2015). Our finding of significantly increased mortality among inactive women with diagnosed EOC supports this hypothesis. We observed no appreciable evidence that this association was confounded or modified by BMI, supporting further investigation of the role that physical inactivity may have in preventing EOC or improving its survivability.
A strength of our study is that our analyses were conducted with individual-level data from well-designed epidemiological investigations. Our ability to adjust for established prognostic factors decreased the chance that the observed associations were explained by confounding. Further, the observed associations remained significant in sensitivity analyses designed to reduce sources of bias. On the other hand, potential measurement error associated with self-reported inactivity data categorised dichotomously is an important limitation. However, using physical inactivity as the exposure variable likely involves less exposure misclassification than would occur with categorised incremental physical activity exposures, and such misclassification would likely be non-differential with respect to vital status, thus tending to bias observed associations toward the null.
In summary, our findings add to a growing body of literature suggesting that physical inactivity is associated with unfavourable health outcomes, including poorer cancer outcomes. Given the global epidemic of physical inactivity, these findings have important public health and clinical implications, particularly in the context of a lack of modifiable prognostic factors for EOC, and only modest improvements in survival among women diagnosed with EOC in recent decades (SEER, 2014). Well-designed prospective studies are needed to confirm the survival benefit and to assess how much mortality can be reduced among women diagnosed with invasive EOC.
Change history
28 June 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Akeson M, Jakobsen AM, Zetterqvist BM, Holmberg E, Brannstrom M, Horvath G (2009) A population-based 5-year cohort study including all cases of epithelial ovarian cancer in western Sweden: 10-year survival and prognostic factors. Int J Gynecol Cancer 19 (1): 116–123.
Bandera EV, King M, Chandran U, Paddock LE, Rodriguez-Rodriguez L, Olson SH (2011) Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study. BMC Womens Health 11: 40.
Berchuck A, Schildkraut JM, Pearce CL, Chenevix-Trench G, Pharoah PD (2008) Role of genetic polymorphisms in ovarian cancer susceptibility: development of an international ovarian cancer association consortium. Adv Exp Med Biol 622: 53–67.
Bodelon C, Cushing-Haugen KL, Wicklund KG, Doherty JA, Rossing MA (2012) Sun exposure and risk of epithelial ovarian cancer. Cancer Causes Control 23 (12): 1985–1994.
Bull FC, Armstrong TP, Dixon T, Ham S, Neiman A, Pratt M (2004) Physical inactivity. In Comparitive Quantification of Health Risks: Global and Regional Burden of Disease Attributable to selected Major Risk Factors WHO(ed) Vol. 1, Chapter 10 World Health Organization: Geneva, Switzerland, pp 729–881.
Byers T (2014) Physical activity and gastric cancer: so what? An epidemiologist’s confession. Cancer Prev Res (Phila) 7 (1): 9–11.
Celis-Morales CA, Perez-Bravo F, Ibanez L, Salas C, Bailey ME, Gill JM (2012) Objective vs self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PloS One 7 (5): e36345.
Cress RD, Chen YS, Morris CR, Petersen M, Leiserowitz GS (2015) Characteristics of long-term survivors of epithelial ovarian cancer. Obstet Gynecol 126 (3): 491–497.
Fiuza-Luces C, Garatachea N, Berger NA, Lucia A (2013) Exercise is the real polypill. Physiology (Bethesda) 28 (5): 330–358.
Glud E, Kjaer SK, Thomsen BL, Hogdall C, Christensen L, Hogdall E, Bock JE, Blaakaer J (2004) Hormone therapy and the impact of estrogen intake on the risk of ovarian cancer. Arch Intern Med 164 (20): 2253–2259.
Goode EL, Chenevix-Trench G, Hartmann LC, Fridley BL, Kalli KR, Vierkant RA, Larson MC, White KL, Keeney GL, Oberg TN, Cunningham JM, Beesley J, Johnatty SE, Chen X, Goodman KE, Armasu SM, Rider DN, Sicotte H, Schmidt MM, Elliott EA, Hogdall E, Kjaer SK, Fasching PA, Ekici AB, Lambrechts D, Despierre E, Hogdall C, Lundvall L, Karlan BY, Gross J, Brown R, Chien J, Duggan DJ, Tsai YY, Phelan CM, Kelemen LE, Peethambaram PP, Schildkraut JM, Shridhar V, Sutphen R, Couch FJ, Sellers TA (2011) Assessment of hepatocyte growth factor in ovarian cancer mortality. Cancer Epidemiol Biomarkers Prev 20 (8): 1638–1648.
Goode EL, Maurer MJ, Sellers TA, Phelan CM, Kalli KR, Fridley BL, Vierkant RA, Armasu SM, White KL, Keeney GL, Cliby WA, Rider DN, Kelemen LE, Jones MB, Peethambaram PP, Lancaster JM, Olson JE, Schildkraut JM, Cunningham JM, Hartmann LC (2010) Inherited determinants of ovarian cancer survival. Clin Cancer Res 16 (3): 995–1007.
Goodman MT, Lurie G, Thompson PJ, McDuffie KE, Carney ME (2008) Association of two common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr Relat Cancer 15 (4): 1055–1060.
Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U (2012) Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet 380 (9838): 247–257.
Hamajima N, Matsuo K, Saito T, Hirose K, Inoue M, Takezaki T, Kuroishi T, Tajima K (2001) Gene-environment interactions and polymorphism studies of cancer risk in the hospital-based epidemiologic research program at Aichi Cancer Center II (HERPACC-II). Asian Pac J Cancer Prev 2 (2): 99–107.
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327 (7414): 557–560.
Hildebrand JS, Gapstur SM, Gaudet MM, Campbell PT, Patel AV (2015) Moderate-to-vigorous physical activity and leisure-time sitting in relation to ovarian cancer risk in a large prospective US cohort. Cancer Causes Control 26 (11): 1691–1697.
Kelemen LE, Goodman MT, McGuire V, Rossing MA, Webb PM, Kobel M, Anton-Culver H, Beesley J, Berchuck A, Brar S, Carney ME, Chang-Claude J, Chenevix-Trench G, Cramer DW, Cunningham JM, Dicioccio RA, Doherty JA, Easton DF, Fredericksen ZS, Fridley BL, Gates MA, Gayther SA, Gentry-Maharaj A, Hogdall E, Kjaer SK, Lurie G, Menon U, Moorman PG, Moysich K, Ness RB, Palmieri RT, Pearce CL, Pharoah PD, Ramus SJ, Song H, Stram DO, Tworoger SS, Van Den Berg D, Vierkant RA, Wang-Gohrke S, Whittemore AS, Wilkens LR, Wu AH, Schildkraut JM, Sellers TA, Goode EL (2010) Genetic variation in TYMS in the one-carbon transfer pathway is associated with ovarian carcinoma types in the Ovarian Cancer Association Consortium. Cancer Epidemiol Biomarkers Prev 19 (7): 1822–1830.
Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J, Deng Y, Chen Q, Nie S, Liu L (2016) The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med 50 (6): 339–345.
Lo-Ciganic WH, Zgibor JC, Bunker CH, Moysich KB, Edwards RP, Ness RB (2012) Aspirin, nonaspirin nonsteroidal anti-inflammatory drugs, or acetaminophen and risk of ovarian cancer. Epidemiology 23 (2): 311–319.
Maldonado G, Greenland S (1993) Simulation study of confounder-selection strategies. Am J Epidemiol 138 (11): 923–936.
McTiernan A (2008) Mechanisms linking physical activity with cancer. Nat Rev Cancer 8 (3): 205–211.
Merritt MA, Green AC, Nagle CM, Webb PM (2008) Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer 122 (1): 170–176.
Risch HA, Bale AE, Beck PA, Zheng W (2006) PGR +331A/G and increased risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 15 (9): 1738–1741.
Rossing MA, Cushing-Haugen KL, Wicklund KG, Doherty JA, Weiss NS (2007) Menopausal hormone therapy and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 16 (12): 2548–2556.
Sanchis-Gomar F, Lucia A, Yvert T, Ruiz-Casado A, Pareja-Galeano H, Santos-Lozano A, Fiuza-Luces C, Garatachea N, Lippi G, Bouchard C, Berger NA (2015) Physical inactivity and low fitness deserve more attention to alter cancer risk and prognosis. Cancer Prev Res (Phila) 8 (2): 105–110.
SEER (2014) SEER Stat Fact Sheets: Ovary Cancer. In SEER Stat Fact Sheets Vol. 2014. National Cancer Institute: Bethesda, MD, USA, Available at http://seer.cancer.gov/statfacts/html/ovary.html.
Terry KL, De Vivo I, Titus-Ernstoff L, Shih MC, Cramer DW (2005) Androgen receptor cytosine, adenine, guanine repeats, and haplotypes in relation to ovarian cancer risk. Cancer Res 65 (13): 5974–5981.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65 (2): 87–108.
UK CR (2015) Ovarian Cancer Statistics. Cancer Research UK: London.
USDHHS (2008) 2008 Physical Activity Guidelines for Americans. Office of Disease Prevention and Health Promotion: Washington, DC, USA.
Winter WE 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F, McGuire WP (2007) Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 25 (24): 3621–3627.
Wright JD, Chen L, Tergas AI, Patankar S, Burke WM, Hou JY, Neugut AI, Ananth CV, Hershman DL (2015) Trends in relative survival for ovarian cancer from 1975 to 2011. Obstet Gynecol 125 (6): 1345–1352.
Wu AH, Pearce CL, Tseng CC, Pike MC (2015) African Americans and Hispanics remain at lower risk of ovarian cancer than Non-Hispanic Whites after considering nongenetic risk factors and oophorectomy rates. Cancer Epidemiol Biomarkers Prev 24 (7): 1094–1100.
Acknowledgements
KBM is supported by NIH/NCI R01CA095023 and NIH/NCI R01CA126841; KHE and KBM were supported by the Roswell Park Alliance Foundation; JBS was supported by 5T32CA108456; ANM was supported by Interdisciplinary Training Grant in Cancer Epidemiology R25CA113951; BHS was supported by R01 CA188900; AUS was supported by the U.S. Army Medical Research and Materiel Command (DAMD17-01-1-0729), National Health & Medical Research Council of Australia, Cancer Councils of New South Wales, Victoria, Queensland, South Australia and Tasmania, Cancer Foundation of Western Australia; National Health and Medical Research Council of Australia (199600 and 400281); CON was supported by National Institutes of Health (R01-CA074850; R01-CA080742); DOV was supported by National Institutes of Health R01-CA112523 and R01-CA87538; HAW was supported by U.S. National Institutes of Health (R01-CA58598, N01-CN-55424 and N01-PC-67001); HOP was supported by DOD: DAMD17-02-1-0669 and NCI: K07-CA080668, R01-CA95023, P50-CA159981, R01-CA126841; JPN was supported by Grant-in-Aid for the Third Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare; MAC was supported by National Institutes of Health (R01-CA122443, P30-CA15083, P50-CA136393); MAL was supported by research grant R01- CA61107 from the National Cancer Institute, Bethesda, MD; research grant 94 222 52 from the Danish Cancer Society, Copenhagen, Denmark; and the Mermaid I project; MAY was supported by National Institutes of Health (R01-CA122443, P30-CA15083, P50-CA136393); Mayo Foundation; Minnesota Ovarian Cancer Alliance; Fred C. and Katherine B. Andersen Foundation; NEC was supported by National Institutes of Health R01-CA54419 and P50-CA105009 and Department of Defense W81XWH-10-1-02802; NJO was supported by National Cancer Institute (NIH-K07 CA095666, R01-CA83918, NIH-K22-CA138563, and P30-CA072720) and the Cancer Institute of New Jersey and NCI CCSG award (P30-CA008748); USC was supported by P01CA17054, P30CA14089, R01CA61132, N01PC67010, R03CA113148, R03CA115195, N01CN025403, and California Cancer Research Program (00-01389V-20170, 2II0200).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
DWC has provided expert testimony for Beasley Allen Crow. MTG is a consultant/advisory board member for Johnson and Johnson. PMW reports receiving a commercial research grant from BUPA. The remaining authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Cannioto, R., LaMonte, M., Kelemen, L. et al. Recreational physical inactivity and mortality in women with invasive epithelial ovarian cancer: evidence from the Ovarian Cancer Association Consortium. Br J Cancer 115, 95–101 (2016). https://doi.org/10.1038/bjc.2016.153
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.153
Keywords
This article is cited by
-
Survival of epithelial ovarian cancer in Black women: a society to cell approach in the African American cancer epidemiology study (AACES)
Cancer Causes & Control (2023)
-
Sedentary behaviour in relation to ovarian cancer risk: a systematic review and meta-analysis
European Journal of Epidemiology (2021)
-
Demand for integrative medicine among women with breast and gynecological cancer: a multicenter cross-sectional study in Southern and Northern Germany
Archives of Gynecology and Obstetrics (2021)
-
Identifying female pelvic cancer survivors with low levels of physical activity after radiotherapy: women with fecal and urinary leakage need additional support
Supportive Care in Cancer (2020)
-
Physical Inactivity and Pancreatic Cancer Mortality
Journal of Gastrointestinal Cancer (2020)