Abstract
Background:
Accurate assessment of the prevalence of the human papilloma virus (HPV) in oropharyngeal tumours (OpSCC) is important because HPV-positive OpSCC are consistently associated with an improved overall survival. Recently, an algorithm has become available that reliably detects clinically relevant HPV in tumour tissue, however, no complete cohorts have been tested. The aim was to determine the prevalence of active high-risk HPV infection in a complete cohort of OpSCC collected over a 16-year period.
Methods:
Using a triple algorithm of p16 immunohistochemistry, HPV-BRISH and HPV-PCR, we assessed the prevalence of active HPV infection in all OpSCC diagnosed in our hospital from 1997 to 2012 (n=193) and a random selection of 200 oral tumours (OSCC).
Results:
Forty-seven OpSCC (24%) were HPVGP PCR-positive; 42 cases were HPV16+, 1 HPV18+, 3 HPV33+ and 1 HPV35+. Brightfield in situ hybridisation did not identify additional HPV-positive cases. Human papilloma virus-associated tumour proportion increased from 13% (1997–2004) to 30% (2005–2012). Human papilloma virus-positivity was an independent predictor for longer disease-specific survival (HR=0.22; 95%CI:0.10–0.47). Only one OSCC was HPV+.
Conclusions:
In our cohort, the incidence of HPV-associated OpSCC is low but increasing rapidly. The strict detection algorithm, analysis of disease-specific survival and the complete cohort, including palliatively treated patients, may influence the reported prevalence and prognostic value of HPV in OpSCC.
Similar content being viewed by others
Main
Infection with the human papilloma virus (HPV) has been identified as a risk factor for the development of various cancer types, such as oropharyngeal cancer, anal cancer, penile cancer and most notably, cervical cancer (Arbyn et al, 2012). In head-neck squamous cell carcinomas (HNSCC), HPV has been detected for almost 30 years (Syrjanen et al, 1983; de Villiers et al, 1985), and was quickly identified as a possible etiological factor (Snijders et al, 1992). In the carcinomas arising in the tonsil and base-of-tongue, two specific sublocations of the oropharynx, a high percentage of HPV positivity is found (Herrero et al, 2003). Both locations harbour lymphatic tissue (resp. palatine and lingual tonsils) and feature a non-keratinised, stratified squamous epithelium with deep invaginations called crypts. These crypts are lined with reticulated epithelium accompanied by disruptions in the basement membrane, which provides direct transepithelial access to antigens (Perry and Whyte, 1998).
Interest in HPV surged when several groups reported a growing incidence of HPV-associated oropharyngeal squamous cell carcinomas (OpSCC) (Frisch et al, 2000; Syrjanen, 2004; Nasman et al, 2009; Marur et al, 2010). The reported prevalence of HPV in OpSCC, however, varies widely. Highest rates are reported in Northern America and Asia (Kreimer et al, 2005), prevalence in Europe is generally reported lower, varying from more than 80% in Stockholm (Nasman et al, 2009; Attner et al, 2010) to 50% in Cologne (Klussmann et al, 2001) and 24% in Amsterdam (Rietbergen et al, 2012). Accurate assessment of the HPV status in these tumours is important because HPV-positive OpSCC are consistently associated with an improved disease-free and overall survival (reported hazard ratios of ∼0.4) (Ragin et al, 2007; Ang et al, 2010; Dayyani et al, 2010). In oral squamous cell carcinomas (OSCC), HPV has not only been reported with a prevalence generally lower than in OpSCC, but also with a wide range, that is, 0%–60%(Kreimer et al, 2005; Isayeva et al, 2012). Moreover, it is not clear whether the effect of HPV status on disease outcome seen in OpSCC is also present in OSCC (Isayeva et al, 2012).
Explanations for the wide range of reported HPV prevalence in HNSCC are differences in geographical and socio-cultural composition of study populations (Kreimer et al, 2005); differences between tumour sublocalisations; between HPV-detection methods and their analytical sensitivity, some of which may also detect transient infections (Braakhuis et al, 2009). Because of the increasing prevalence, different study periods are not readily comparable (Mehanna et al, 2013) and the proportion of positivity depends on the incidence of non-HPV-associated HNSCC.
Several algorithms have been proposed to reliably detect active HPV infection in HNSCC (Smeets et al, 2007; Braakhuis et al, 2009; Thavaraj et al, 2011). Currently used algorithms consist of p16 immunohistochemistry, followed by HPV-PCR of the p16-positive cases. Recently, in situ hybridisation (ISH) has been added to these algorithms because of its high specificity and, therefore, the ability to confirm p16-negative cases (Smeets et al, 2007; Pannone et al, 2012).
Currently, very little information is available on regional differences in the prevalence of HPV-associated OpSCC. Even within one country, reported prevalence may differ widely. In the southern and central parts of the Netherlands, several studies into HPV prevalence in OpSCC have been performed. Prevalence rates in these studies range from 24% to 65% (Smeets et al, 2007; Hafkamp et al, 2008; Rietbergen et al, 2012; van Monsjou et al, 2012). Previously, we identified only two HPV-positive cases in a series of 140 advanced HNSCCs from the Northern Netherlands (Pattje et al, 2010). However, no complete cohorts have been tested. Human papilloma virus prevalence in the Northern Netherlands may be different because of more smokers and a higher ratio of elderly people (Statistics Netherlands, 2013).
Therefore, the aim of this study was to determine the incidence of high-risk HPV-associated tumours in oropharyngeal and OSCCs from the Northern Netherlands and to validate whether HPV-positive tumours exhibit a clinically different behaviour compared with HPV-negative tumours. For this purpose, we collected a complete 16-year cohort of tonsillar and base-of-tongue squamous cell carcinomas (OpSCC), and a representative group of OSCC, for which complete clinicopathological and follow-up data were available, and determined the presence of active high-risk HPV infection in tumour tissue using a validated detection algorithm.
Methods
Patient selection
From the database of the Netherlands Cancer Registry, all records with the following criteria were retrieved: base-of-tongue and tonsillar primary tumour location (ICD-O-3 locations C01.9 and C09, respectively), histologically proven squamous cell carcinoma and diagnosed from 1 January 1997 until 31 December 2012. All patients were diagnosed in the UMC Groningen, and biopsy or resection material was available in our archives.
All tissue blocks and original haematoxylin and eosin slides were retrieved from the archives of our department. The histopathological diagnoses were revised by a head-neck pathologist. Eight out of 128 (6%) tonsillar and 8 out of 81 (11%) of the base-of-tongue cases did not have sufficient tumour material available for analysis, and were excluded, leaving 193 OpSCC cases.
Additionally, 200 OSCCs were selected. Clinicopathological and follow-up data have been published recently (Melchers et al, 2013). Twenty-four OSCC cases did not have sufficient tumour cells available for analysis or had insufficient DNA quality. These cases were excluded from further analysis, leaving 176 OSCC cases.
HPV testing is not routinely performed on OpSCC or OSCC in our institution, as treatment choice according to national treatment guidelines is independent from HPV status.
Tissue microarray construction
For the OpSCC cases, five separate tissue microarrays (TMAs) were constructed from the tumour centre as described recently (Melchers et al, 2013). Twenty OpSCC cases were considered too small to reliably include in TMA construction. Full sections of these cases were included in the study. After TMA construction, 3-μm thick sections were cut (4 μm sections for BRISH), performing haematoxylin-eosin staining on the first section to confirm the quality of the TMA and the presence of tumour cells in each core. The OSCC samples were distributed on five TMAs, as described previously (Melchers et al, 2013).
Human papilloma virus testing algorithm
To determine which tumours harbour high-risk HPV, we used a triple algorithm that detects active HPV infection in tumour tissue (Figure 1). All cases that showed positive expression of p16 and also showed presence of high-risk HPV-DNA as determined by PCR were considered HPV-positive for active high-risk HPV. Human papilloma virus-BRISH was performed on all available cases, but was only relevant on p16-negative cases: p16-negative cases that were BRISH-positive were additionally tested by HPV-PCR. Thus, BRISH was used as an extra control for p16-negative cases.
p16 immunohistochemistry and analysis
Immunohistochemistry for p16 was performed using the CINtec Histology kit (Roche mtm laboratories AG, Heidelberg, Germany), according to the manufacturer’s protocol (mtm laboratories AG, 2007). Cervical carcinoma tissue was used as the positive control. All slides were analyzed independently by two observers. Discordant cases were discussed with an experienced head-neck pathologist until consensus was reached. Moderate or strong nuclear and cytoplasmic staining of ≥70% of the tumour cells was considered ++ expression (Ang et al, 2010). Because lower percentages are frequently used as cut-off (van Monsjou et al, 2012) and are relevant for HPV detection (Lewis et al, 2012), we considered moderate or strong staining of <70% of tumour cells+expression. For this study, both ++ and +expressing cases were considered p16-positive (Figure 2). Thus, increasing the sensitivity of the p16 analysis, and reducing the chance for false-negative result in the first step of testing algorithm. All other staining patterns were considered negative.
Human papilloma virus-BRISH
HPV brightfield in situ hydridisation was performed automated on a Ventana Benchmark Ultra machine (Ventana-Roche, Basel, Switzerland), using the commercially available Inform HPV III family 16 probe kit. This kit contains a probe cocktail with reported hybridisation to 12 high-risk HPV-genotypes: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 66. Slides were developed with the ISH iVIEW Blue detection kit, according to the manufacturer’s protocol. Ventana’s HPV 3 in 1 control slide was used, containing Caski (HPV16) and HeLa (HPV18) and C-33A (HPV-negative) cell lines. All products were purchased from Ventana-Roche (Basel, Switzerland). Cases were evaluated by scoring the percentage of tumour cells with any of three patterns of signals: either punctuate (clear dotted nuclear signal), granular (large, irregular, inhomogeneous signal) or a combination of these patterns (Thavaraj et al, 2011; Pannone et al, 2012). Cases harbouring these signals in >3% of tumour cells were considered HPV-BRISH-positive. All other staining patterns were considered negative.
Human papilloma virus-PCR
For all cases that were considered p16-positive and/or HPV-BRISH-positive, two 10-μm thick sections were cut from the original FFPE blocks. Every case was cut using a new microtome blade to prevent contamination. Two 20-μm sections were cut from an empty paraffin block prior to every tissue block and were analyzed in parallel with every case as negative control, to ensure no cross contamination had occurred. After deparaffinisation, DNA isolation was performed, using standard salt-chloroform extraction and ethanol precipitation. For quality control, genomic DNA was amplified in a multiplex PCR containing a control gene primer set resulting in products of 100, 200, 300, 400 and 600 bp according to the BIOMED-2 protocol (van Dongen et al, 2003). Cases with a product <200 bp were excluded from further analysis. All samples and adjoining empty paraffin controls were analyzed for the presence of high-risk HPV-DNA with both the HPVGP5+/6+ general primers, and HPV16-specific primers (Wisman et al, 2006), as performed routinely in our ISO-15189 accredited laboratory. For every PCR, the following controls were included: CaSki (HPV16 high copy), SiHa (HPV16 low copy), HeLa (HPV18), CC10B (HPV45), CC11 (HPV67) and BSM (HPV−). For every HPVGP PCR, positive controls were included in undiluted, 1/10, 1/100 and 1/1000 concentrations. For the HPV16-specific-PCR the same controls were included, as positive (CasKi, SiHa) or negative control (HeLa, CC10B, CC11 and BSM). Every PCR thus had a minimal analytical sensitivity of 1/1000 SiHa cells, which contain ∼2 copies HPV16 per cell. Samples showing a product that was quantified weaker than SiHa 1/1000, were performed in duplicate, and, if discordant in triplicate to obtain a definitive result.
All HPVGP-positive cases, which were negative for HPV16- and HPV18-specific PCR, were genotyped using Sanger sequence analysis as reported previously (Krul et al, 1999). The HPVGP product was sequenced using the ‘quick shot’ method (Baseclear, Leiden, The Netherlands). Sequences were compared and aligned with sequences present in the EMBL database (http://www.ebi.ac.uk/Tools/sss/wublast/nucleotide.html). The HPV type with >95% sequence similarity was considered present.
Statistical analysis
Statistical analysis was performed with PASW Statistics 20.0 (IBM software, Armonk, NY, USA). Categorical variables were compared with HPV status (positive or negative) using the Chi-square test or Fisher exact test, when appropriate. Tumour incidence and the proportion of HPV-positive tumours were calculated over time and a trend was evaluated by univariate linear regression analysis. Multiple logistic regression analysis was used to assess HPV-associated tumour incidence over time, adjusted for clinicopathological variables. Kaplan–Meier curves were created to visualise survival differences and the log rank test was used to compare survival between HPV-positive and -negative groups. Univariate and multiple Cox regression were used to assess the relationship between predictor variables and survival. Multiple regression analyses were performed backward stepwise, and included all variables with P<0.10 in univariate regression. Survival was defined as time from first treatment until last follow-up or disease-specific death (disease-specific survival) or till disease recurrence (disease-free survival). Tests were performed two-tailed. P<0.05 was considered statistically significant.
Results
Patient characteristics
A complete cohort of 120 tonsillar and 73 base-of-tongue cases (193 OpSCC) was included in this study (Table 1). Of the patients, 64% was male, median age was 58 years and 86% was former or current smoker; 81% was treated with curative intent. During follow-up (median 29 months), 57 cases developed a recurrence and 76 cases died of disease. Additionally, 176 OSCC were included in this study (Table 1). Of the patients, 60% was male, median age was 63 years. Most tumours were located in the tongue (34%) or floor of mouth (40%). During follow-up (median 45 months), 33 cases developed a recurrence. Forty-one cases died of disease.
Human papilloma virus testing of tonsillar and base-of-tongue tumours
p16 immunohistochemistry was performed on 120 tonsillar and 73 base-of-tongue tumours. Sixty-four (33%) were considered p16-positive. Further analysis by HPV-PCR revealed 47 cases as HPVGP-positive. Of the 47 HPVGP-positive cases, 42 were found to be HPV16-positive. Sequence analysis of the five HPVGP-positive/HPV16-negative cases revealed HPV33 in three cases, HPV35 in one and HPV18 in one (Figure 1). Human papilloma virus-BRISH was performed as extra control for p16-negative cases. Of the 181 cases that could be analyzed 38 (21%) were considered BRISH-positive. Most (n=36) were also positive for p16 and HPV-PCR (Table 3). Only two cases were BRISH-positive but p16-negative, and additional analysis by HPV-PCR revealed HPV-negativity (Figure 1). No associations were observed between BRISH pattern and HPV status (data not shown).
The overall proportion of active high-risk HPV infection in tonsillar and base-of-tongue tumours during our study period 1997–2012 was 24%. Notably, the proportion of HPV-positive tumours increased from 13% (8/62) during the first half (1997–2004) to 30% (39/131) during the second half (2005–2012) of the study period. There was a significant increase of both the absolute number as well as the proportion of HPV-positive tumours throughout the research period (P=0.001 and P=0.01, respectively; Figure 3).
HPV-positivity was significantly associated with younger age, the absence of a smoking history, lower cT and pT status, curatively intended treatment, and longer loco-regional disease-free survival and disease-specific survival (Table 2). When adjusted for age, smoking history, cT and pT status, and treatment intent, there was still a significant increase of HPV-positive tumours throughout time (P=0.02), as assessed by multiple logistic regression analysis (data not shown).
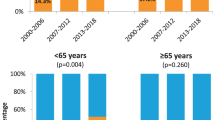
Disease-specific survival of patients with HPV-positive tumours was significantly better than that of patients with HPV-negative tumours (P<0.001). Five-year disease-specific survival was 78% in the HPV-positive group versus 43% in the HPV-negative group (Figure 4). Because HPV status and survival are both associated with treatment intent (curative or palliative), Cox regression was performed separately on all cases (n=193) and on curatively treated cases only (n=156). Cox regression included the variables sex, age at diagnosis, smoking history, tumour site, cT and cN status, and HPV status (Table 4). Sex and smoking history were not significant predictors for disease-specific survival. Subsequent multiple Cox regression identified cN status and HPV status as independent predictors for disease-specific survival both in all cases (Table 4A) and in the curatively treated cases only (Table 4B).
For loco-regional-disease-free survival, Cox regression was performed only on curatively treated cases (n=156), including the same variables as for disease-specific survival. Tumour site (trend) and HPV status (significant) were univariate predictors. In multiple Cox regression analysis, HPV status was the only independent significant predictor for loco-regional-disease-free survival (Table 4C).
Human papilloma virus testing of oral tumours
p16 staining was performed on 176 OSCCs (Table 1). Twenty out of 176 (11%) were considered p16-positive, and were further analyzed by HPV-PCR. As for the OpSCC, all p16-positive cases were also tested by HPV16-specific PCR which was negative for all cases. One out of 20 was HPVGP-positive. By sequencing, the single HPVGP-positive case was identified as HPV33-positive (Figure 1). This tumour was localised in the border of tongue. The patient was treated with curative intent and did not develop recurrences. Human papilloma virus-BRISH could be analyzed on 175 cases, and revealed 173 (99%) BRISH-negative cases. Only two cases were considered HPV-BRISH-positive. Both cases also were p16-positive and thus tested by HPV-PCR. The single HPV33-positive case was one of these. The other case was HPV-negative.
Discussion
We set out to study the prevalence of HPV in two well-defined cohorts of head-neck tumours. Using a validated algorithm, we analyzed the presence of active, high-risk HPV infection in OpSCC (a complete retrospective cohort of tonsillar and base-of-tongue tumours, n=193) and one consisting of OSCCs (n=176).
Twenty-four percent (47 cases) of OpSCC were HPV-positive. A significant increase during the study period 1997–2012 was seen, with 13% HPV-positive cases during the first half (1997–2004) increasing to 30% HPV-positive during the second half (2005–2012). Only one case (<1%) in our series of OSCC was HPV-positive.
The percentage of HPV-positive OpSCC in our series of 24% is low, compared with 39.7% reported in a recent meta-analysis of 2278 European OpSCC cases (Mehanna et al, 2013). This difference may be due to several reasons.
First, we used a combination of detection techniques of p16 immunohistochemistry, HPV-BRISH and HPV-PCR, which has been shown to have the most optimal sensitivity and specificity to detect clinically relevant active high-risk HPV infection (Smeets et al, 2007; Thavaraj et al, 2011; Pannone et al, 2012). Most studies on HPV prevalence still use a single technique of PCR or ISH (Dayyani et al, 2010; O'Rorke et al, 2012; Mehanna et al, 2013). Performing only PCR may result in overdetection of clinically irrelevant HPV (Smeets et al, 2007). Performing only ISH may result in underdetection (Table 3) (Lewis et al, 2010; Schache et al, 2011; Pannone et al, 2012). p16 immunohistochemistry has been added to these protocols because it detects transcriptionally active HPV with a high sensitivity of up to 100% (Smeets et al, 2007; Pannone et al, 2012). A meta-analysis by Mehanna et al (2013) identified only 14 out of 269 studies that assessed OpSCC using a combination of p16 and PCR/ISH, 5 of which were European cohorts (Hafkamp et al, 2003; Paradiso et al, 2004; Licitra et al, 2006; Reimers et al, 2007; O'Regan et al, 2008). The four studies that provided separate data on p16 and HPV-DNA detection (PCR technique in three, FISH in one) (Hafkamp et al, 2003; Licitra et al, 2006; Reimers et al, 2007; O'Regan et al, 2008), analyzed 213 OpSCC, of which 58 (27%) were p16-positive and PCR/ISH-positive. The similarity with the data from our series illustrates the large effect that detection technique may have on the reported HPV prevalence.
A second reason why the proportion of HPV-positive tumours is lower in our series compared with literature is that we analyzed a complete cohort of all patients diagnosed with OpSCC in our hospital over a 16-year period. This also includes palliatively treated cases. In literature, patient selection criteria are not always clear and when reported, exclusion of palliatively treated cases is common (Reimers et al, 2007; Settle et al, 2009; Pannone et al, 2012; Cerezo et al, 2013). Because we showed that palliatively treated cases are more frequently HPV-negative (Table 2), exclusion of these cases may severely overestimate HPV prevalence. When excluding palliatively treated cases, the proportion of HPV-associated tumours in our study would increase from 24 to 28% (Table 2).
The univariate HR of 0.22 for disease-specific survival for HPV-positive patients in our series is low, but the 95%CI of 0.10–0.47 includes the HR for overall survival of ∼0.4 that is generally reported (Ragin and Taioli, 2007; Dayyani et al, 2010; O'Rorke et al, 2012). The HR found in our series may again be attributed our strict detection algorithm. Suboptimal detection techniques would cause both false-positive and false-negative detection of clinically relevant HPV and thus result in a dilution of the survival difference between HPV-positive and -negative groups. Exclusion of palliatively treated cases slightly increases the HR for disease-specific death, both in univariate as well as in multiple regression analysis (Table 4A and B). Moreover, we analyzed disease-specific rather than overall survival, because risk factor profiles of HPV-negative patients might not compare with that of HPV-positive patients, who are generally younger and drink and smoke less (Hafkamp et al, 2008; Nasman et al, 2009; Ang et al, 2010; Schache et al, 2011; Chaturvedi, 2012; Rietbergen et al, 2012), with subsequent influence on the overall survival. For comparison purposes only, HPV-positivity was associated with a univariate HR for overall survival of 0.27; 95%CI: 0.14–0.52 (n=193). When the palliatively treated cases (mostly HPV-negative tumours; Table 2) are excluded, this ratio further increases to HR=0.30; 95%CI: 0.14–0.63.
These examples clearly indicate the influence that detection technique, population selection criteria and type of survival analysis may have on both reported prevalence and prognostic value of HPV in OpSCC.
All patients in our study were treated in the University Medical Center Groningen, which is situated in the North of the Netherlands and is one of the eight head-neck oncology centres in the country. Prevalence of HPV in head-neck tumours has not been studied yet in the Northern part of the Netherlands. Moreover, no complete cohorts have ever been tested to determine HPV prevalence in OpSCC in the Netherlands. Two studies were performed in medical centres in Amsterdam, using a combination of p16 immunohistochemistry and PCR techniques. No BRISH was used. One of these was a very small study (18 OpSCC), and selection criteria were not described (Smeets et al, 2007). The other study was larger and included 150 tonsillar and base-of-tongue SCC, but selection criteria are again not clear. Cases from five separate years over a 20-year span were analyzed (Rietbergen et al, 2012). Finally, another study was performed on 81 tonsillar SCCs from the Southern part of the Netherlands, using only p16 immunohistochemistry and HPV16-specific FISH detection (Hafkamp et al, 2008) (Supplementary Data). The heterogeneity in selection criteria and testing methods makes comparison with the current study impossible.
Of the 176 oral SCCs analyzed, we found only one HPV-positive case (<1%). Also, in literature, HPV-positive OSCC rates are much lower than in OpSCC. Kreimer et al (2005) reported an overall prevalence of 16.0% in 15 European studies. A large international study identified HPV DNA in 3.9% of 766 OSCCs (Herrero et al, 2003). Misclassification of OpSCC as OSCC is a possible explanation (Herrero et al, 2003; Kreimer et al, 2005). Moreover, HPV DNA has also been found present in the oral cavity of 3.7% of 5579 healthy US citizens (Gillison et al, 2012), indicating that assessing tumour HPV status with PCR alone may grossly overestimate clinically relevant HPV infection.
The current study assessed HPV status by a combined triple algorithm of p16 immunohistochemistry, high-risk HPV PCR and BRISH. In situ hybridisation techniques have been added to HPV detection protocols (Lewis et al, 2010; Thavaraj et al, 2011; Pannone et al, 2012), because of their high specificity of 95–100% (Smeets et al, 2007; Jordan et al, 2012). In our study, BRISH specificity was 99%. Most importantly, however, BRISH did not identify any additional HPV-positive cases that were not identified by p16 immunohistochemistry. Therefore, the current BRISH technique has no additional benefit to p16 combined with HPV-PCR for the detection of HPV in our series of OpSCC.
For p16 expression, we considered all cases with any moderate to strong expression as p16-positive. This way, we identified 64 p16-positive cases, of which 47 were HPV-positive, resulting in a positive predictive value of 73%. Most recent studies, however, choose a threshold of ≥70% moderate to strong expression as p16-positive. In our series, this threshold would have resulted in an increased positive predictive value of 85%, but two false-negative (p16-negative/PCR-positive) cases. These cases were also BRISH-negative and therefore would not be detected with a p16 threshold of 70%, even in a triple detection algorithm such as was used in the current study.
In conclusion, in our complete cohort 1997–2012 of tonsillar and base-of-tongue squamous cell carcinomas from the Northern Netherlands, there is evidence for a rapid increase of the incidence of HPV-associated OpSCC. In our series of OSCCs, HPV is rare and therefore seems of minimal clinical relevance. This study illustrates the influence that detection technique, population selection criteria and type of survival analysis may have on both reported prevalence and prognostic value of HPV in OpSCC.
Change history
14 April 2015
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363: 24–35.
Arbyn M, de Sanjose S, Saraiya M, Sideri M, Palefsky J, Lacey C, Gillison M, Bruni L, Ronco G, Wentzensen N, Brotherton J, Qiao YL, Denny L, Bornstein J, Abramowitz L, Giuliano A, Tommasino M, Monsonego J (2012) EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J Cancer 131: 1969–1982.
Attner P, Du J, Nasman A, Hammarstedt L, Ramqvist T, Lindholm J, Marklund L, Dalianis T, Munck-Wikland E (2010) The role of human papillomavirus in the increased incidence of base of tongue cancer. Int J Cancer 126: 2879–2884.
Braakhuis BJ, Brakenhoff RH, Meijer CJ, Snijders PJ, Leemans CR (2009) Human papilloma virus in head and neck cancer: the need for a standardised assay to assess the full clinical importance. Eur J Cancer 45: 2935–2939.
Cerezo L, Lopez C, de la Torre A, Suarez D, Hervas A, Ruiz A, Ballestin C, Martin M, Sandoval P (2013) Incidence of HPV-related oropharyngeal cancer and outcomes after chemoradiation in a population of heavy smokers. Head Neck 36 (6): 782–786.
Chaturvedi AK (2012) Epidemiology and clinical aspects of HPV in head and neck cancers. Head Neck Pathol 6 (Suppl 1): S16–S24.
Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS (2010) Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol 2: 15.
de Villiers EM, Weidauer H, Otto H, zur Hausen H (1985) Papillomavirus DNA in human tongue carcinomas. Int J Cancer 36: 575–578.
Frisch M, Hjalgrim H, Jaeger AB, Biggar RJ (2000) Changing patterns of tonsillar squamous cell carcinoma in the United States. Cancer Causes Control 11: 489–495.
Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, Graubard BI, Chaturvedi AK (2012) Prevalence of oral HPV infection in the United States, 2009-2010. JAMA 307: 693–703.
Hafkamp HC, Manni JJ, Haesevoets A, Voogd AC, Schepers M, Bot FJ, Hopman AH, Ramaekers FC, Speel EJ (2008) Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer 122: 2656–2664.
Hafkamp HC, Speel EJ, Haesevoets A, Bot FJ, Dinjens WN, Ramaekers FC, Hopman AH, Manni JJ (2003) A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5-8. Int J Cancer 107: 394–400.
Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, Balaram P, Rajkumar T, Sridhar H, Rose B, Pintos J, Fernandez L, Idris A, Sanchez MJ, Nieto A, Talamini R, Tavani A, Bosch FX, Reidel U, Snijders PJ, Meijer CJ, Viscidi R, Munoz N, Franceschi S IARC Multicenter Oral Cancer Study Group (2003) Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 95: 1772–1783.
Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M (2012) Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol 6 (Suppl 1): S104–S120.
Jordan RC, Lingen MW, Perez-Ordonez B, He X, Pickard R, Koluder M, Jiang B, Wakely P, Xiao W, Gillison ML (2012) Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol 36: 945–954.
Klussmann JP, Weissenborn SJ, Wieland U, Dries V, Kolligs J, Jungehuelsing M, Eckel HE, Dienes HP, Pfister HJ, Fuchs PG (2001) Prevalence, distribution, and viral load of human papillomavirus 16 DNA in tonsillar carcinomas. Cancer 92: 2875–2884.
Kreimer AR, Clifford GM, Boyle P, Franceschi S (2005) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 14: 467–475.
Krul EJ, Van De Vijver MJ, Schuuring E, Van Kanten RW, Peters AA, Fleuren GJ (1999) Human papillomavirus in malignant cervical lesions in Surinam, a high-risk country, compared to the Netherlands, a low-risk country. Int J Gynecol Cancer 9: 206–211.
Lewis JS Jr, Chernock RD, Ma XJ, Flanagan JJ, Luo Y, Gao G, Wang X, El-Mofty SK (2012) Partial p16 staining in oropharyngeal squamous cell carcinoma: extent and pattern correlate with human papillomavirus RNA status. Mod Pathol 25: 1212–1220.
Lewis JS Jr, Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q, El-Mofty SK (2010) p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol 34: 1088–1096.
Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R, Oggionni M, Rossini C, Cantu G, Squadrelli M, Quattrone P, Locati LD, Bergamini C, Olmi P, Pierotti MA, Pilotti S (2006) High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 24: 5630–5636.
Marur S, D'Souza G, Westra WH, Forastiere AA (2010) HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 11: 781–789.
Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, Roberts S (2013) Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—systematic review and meta-analysis of trends by time and region. Head Neck 35: 747–755.
Melchers LJ, Bruine de Bruin L, Schnell U, Slagter-Menkema L, Mastik MF, de Bock GH, van Dijk BAC, Giepmans BNG, van der Laan BFAM, van der Wal JE, Roodenburg JLN, Schuuring E (2013) Lack of claudin-7 is a strong predictor of regional recurrence in oral and oropharyngeal squamous cell carcinoma. Oral Oncol 49: 998–1005.
mtm laboratories AG (2007) Instructions for use CINtec Histology Kit, ref.9511.
Nasman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, Ahrlund-Richter S, Marklund L, Romanitan M, Lindquist D, Ramqvist T, Lindholm J, Sparen P, Ye W, Dahlstrand H, Munck-Wikland E, Dalianis T (2009) Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer 125: 362–366.
O'Regan EM, Toner ME, Finn SP, Fan CY, Ring M, Hagmar B, Timon C, Smyth P, Cahill S, Flavin R, Sheils OM, O'Leary JJ (2008) p16(INK4A) genetic and epigenetic profiles differ in relation to age and site in head and neck squamous cell carcinomas. Hum Pathol 39: 452–458.
O'Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA (2012) Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol 48: 1191–1201.
Pannone G, Rodolico V, Santoro A, Lo Muzio L, Franco R, Botti G, Aquino G, Pedicillo MC, Cagiano S, Campisi G, Rubini C, Papagerakis S, De Rosa G, Tornesello ML, Buonaguro FM, Staibano S, Bufo P (2012) Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 Immunohistochemistry, Consensus PCR HPV-DNA, and In Situ Hybridization. Infect Agent Cancer 7: 4.
Paradiso A, Ranieri G, Stea B, Zito A, Zehbe I, Tommasino M, Grammatica L, De Lena M (2004) Altered p16INK4a and Fhit expression in carcinogenesis and progression of human oral cancer. Int J Oncol 24: 249–255.
Pattje WJ, Schuuring E, Mastik MF, Slagter-Menkema L, Schrijvers ML, Alessi S, van der Laan BF, Roodenburg JL, Langendijk JA, van der Wal JE (2010) The phosphatase and tensin homologue deleted on chromosome 10 mediates radiosensitivity in head and neck cancer. Br J Cancer 102: 1778–1785.
Perry M, Whyte A (1998) Immunology of the tonsils. Immunol Today 19: 414–421.
Ragin CC, Modugno F, Gollin SM (2007) The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res 86: 104–114.
Ragin CC, Taioli E (2007) Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer 121: 1813–1820.
Reimers N, Kasper HU, Weissenborn SJ, Stutzer H, Preuss SF, Hoffmann TK, Speel EJ, Dienes HP, Pfister HJ, Guntinas-Lichius O, Klussmann JP (2007) Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer 120: 1731–1738.
Rietbergen MM, Leemans CR, Bloemena E, Heideman DA, Braakhuis BJ, Hesselink AT, Witte BI, de Jong RJ, Meijer CJ, Snijders PJ, Brakenhoff RH (2012) Increasing prevalence rates of HPV attributable oropharyngeal squamous cell carcinomas in the Netherlands as assessed by a validated test algorithm. Int J Cancer 132 (7): 1565–1571.
Schache AG, Liloglou T, Risk JM, Filia A, Jones TM, Sheard J, Woolgar JA, Helliwell TR, Triantafyllou A, Robinson M, Sloan P, Harvey-Woodworth C, Sisson D, Shaw RJ (2011) Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin Cancer Res 17: 6262–6271.
Settle K, Posner MR, Schumaker LM, Tan M, Suntharalingam M, Goloubeva O, Strome SE, Haddad RI, Patel SS, Cambell EV 3rd, Sarlis N, Lorch J, Cullen KJ (2009) Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila) 2: 776–781.
Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, Meijer CJ, Braakhuis BJ, Leemans CR, Brakenhoff RH (2007) A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer 121: 2465–2472.
Snijders PJ, Cromme FV, van den Brule AJ, Schrijnemakers HF, Snow GB, Meijer CJ, Walboomers JM (1992) Prevalence and expression of human papillomavirus in tonsillar carcinomas, indicating a possible viral etiology. Int J Cancer 51: 845–850.
Statistics Netherlands (2013) Population data, age, region and lifestyle. 2013.
Syrjanen K, Syrjanen S, Lamberg M, Pyrhonen S, Nuutinen J (1983) Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg 12: 418–424.
Syrjanen S (2004) HPV infections and tonsillar carcinoma. J Clin Pathol 57: 449–455.
Thavaraj S, Stokes A, Guerra E, Bible J, Halligan E, Long A, Okpokam A, Sloan P, Odell E, Robinson M (2011) Evaluation of human papillomavirus testing for squamous cell carcinoma of the tonsil in clinical practice. J Clin Pathol 64: 308–312.
van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E, Garcia-Sanz R, van Krieken JH, Droese J, Gonzalez D, Bastard C, White HE, Spaargaren M, Gonzalez M, Parreira A, Smith JL, Morgan GJ, Kneba M, Macintyre EA (2003) Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 17: 2257–2317.
van Monsjou HS, van Velthuysen ML, van den Brekel MW, Jordanova ES, Melief CJ, Balm AJ (2012) Human papillomavirus status in young patients with head and neck squamous cell carcinoma. Int J Cancer 130: 1806–1812.
Wisman GB, Nijhuis ER, Hoque MO, Reesink-Peters N, Koning AJ, Volders HH, Buikema HJ, Boezen HM, Hollema H, Schuuring E, Sidransky D, van der Zee AG (2006) Assessment of gene promoter hypermethylation for detection of cervical neoplasia. Int J Cancer 119: 1908–1914.
Acknowledgements
We would like to thank J. Holewijn for his help in performing the HPV testing. This work was partly funded by the CTMM Air Force consortium (http://www.ctmm.nl). CTMM pays for part of the salary of MFM and had no role in study design, data collection and analysis, decision to publish and preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Melchers, L., Mastik, M., Samaniego Cameron, B. et al. Detection of HPV-associated oropharyngeal tumours in a 16-year cohort: more than meets the eye. Br J Cancer 112, 1349–1357 (2015). https://doi.org/10.1038/bjc.2015.99
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.99
Keywords
This article is cited by
-
Association of Human Papillomavirus Infection with Tonsillar Cancers: A Systematic Review
Indian Journal of Otolaryngology and Head & Neck Surgery (2024)
-
Global prevalence of human papillomavirus-related oral and oropharyngeal squamous cell carcinomas: a systematic review and meta-analysis
Clinical Oral Investigations (2023)
-
Prevention of HPV-Related Oral Cancer by Dentists: Assessing the Opinion of Dutch Dental Students
Journal of Cancer Education (2018)
-
FGFR Family Members Protein Expression as Prognostic Markers in Oral Cavity and Oropharyngeal Squamous Cell Carcinoma
Molecular Diagnosis & Therapy (2016)