Abstract
Background:
This randomised phase II study evaluated the efficacy and safety of panitumumab added to docetaxel-based chemotherapy in advanced oesophagogastric cancer.
Methods:
Patients with metastatic or locally recurrent cancer of the oesophagus, oesophagogastric junction or stomach received docetaxel and a fluoropyrimidine with or without panitumumab for 8 cycles or until progression. The primary end point was response rate (RECIST1.1). We planned to enrol 100 patients, with 50% expected response rate for combination therapy.
Results:
A total of 77 patients were enrolled. A safety alert from the REAL3 trial prompted a review of data that found no evidence of adverse outcomes associated with panitumumab but questionable efficacy, and new enrolment was ceased. Enrolled patients were treated according to protocol. Response rates were 49% (95% CI 34–64%) in the chemotherapy arm and 58% (95% CI 42–72%) in the combination arm. Common grade 3 and 4 toxicities included infection, anorexia, vomiting, diarrhoea and fatigue. At 23.7 months of median follow-up, median progression-free survival was 6.9 months vs 6.0 months and median overall survival was 11.7 months vs 10.0 months in the chemotherapy arm and the combination arm, respectively.
Conclusions:
Adding panitumumab to docetaxel-based chemotherapy for advanced oesophagogastric cancer did not improve efficacy and increased toxicities.
Similar content being viewed by others
Main
Most cases of oesophageal and gastric cancer present with locally advanced or metastatic disease. Median overall survival is <6 months for those who receive best supportive care alone for metastatic disease (Murad et al, 1993; Glimelius et al, 1997). Cisplatin and fluorpyrimidine combination regimens are widely accepted as standard therapy for this disease (Wagner et al, 2006).
Recently, the REAL-2 study demonstrated that capecitabine is not inferior to 5-fluorouracil in this setting (Cunningham et al, 2008). This alternative is easier to administer and obviates indwelling venous access.
Docetaxel has promising activity in advanced gastro-oesophageal cancer, as a single agent and in combination regimens (Muro et al, 2004; Van Cutsem et al, 2006; Cook et al, 2013). Our group showed that a docetaxel-based chemotherapy regimen given weekly (in combination with a fluoropyrimidine or fluoropyrimidine plus cisplatin) has comparable activity to 3-weekly administration, with a lower rate of haematological toxicity (Tebbutt et al, 2010). The triplet regimen appeared to have the most promising activity, leading to its incorporation into this subsequent trial.
The EGFR pathway is important to development of a number of tumour types, and has become a target for therapy in some gastrointestinal cancers, including colorectal cancer (Jonker et al, 2007; Van Cutsem et al, 2007). Strong EGFR expression has been found in epidermal oesophageal cancers (Langer et al, 2006), with some evidence that EGFR expression predicts poor prognosis (Lieto et al, 2008; Navarini et al, 2012). Oesophagogastric cancers have low rates of ras mutation, suggesting that targeting of this pathway may add to the benefit received from chemotherapy alone. We have shown that the addition of an EGFR-targeted therapy to docetaxel achieves modest response rates in the docetaxel-refractory setting (Tebbutt et al, 2013).
Therefore, this study evaluated the activity of the combination of docetaxel-based weekly chemotherapy with the EGFR human monoclonal antibody panitumumab as first-line treatment of advanced oesophagogastric cancer.
Materials and methods
Eligibility
The AGITG ATTAX3 study was approved by the research ethics committee of each participating institution. All patients provided written informed consent.
The ATTAX3 study was available for adult patients with histologically proven metastatic or locally recurrent cancer of the oesophagus, oesophagogastric junction or stomach (squamous, adenocarcinoma or undifferentiated histology), who had received no prior therapy other than neoadjuvant or adjuvant treatment completed at least 12 months earlier or palliative radiotherapy completed at least 14 days before enrolment. Other inclusion criteria included WHO performance status (PS) 0, 1 or 2; adequate haematological function, including platelets >100 × 109 l−1 and neutrophils >1.5 × 109 l−1; normal renal function, including calculated creatinine clearance of at least 50 ml min−1; and adequate hepatic function, including serum total bilirubin <1.25 × upper limit of normal range, alanine transaminase (ALT) or aspartate transaminase (AST) <2.5 × upper limit of normal range and alkaline phosphatase <5 × upper limit of normal range. Women of childbearing potential were required to have a negative pregnancy test and adequate contraception. Exclusion criteria included medical or psychiatric conditions that compromised a patient’s ability to consent to or comply with the study requirements; previous malignant disease other than nonmelanotic skin cancer, carcinoma in situ of the uterine cervix or other cancer treated with curative intent at least 2 years previously and without evidence of relapse; uncontrolled metastatic disease of the central nervous system; pregnancy or breast-feeding; clinical evidence of peripheral neuropathy >grade 2; and clinical evidence of interstitial pneumonitis or pulmonary fibrosis. Patients with any uncontrolled concurrent medical condition or who had previously used an EGFR-targeted agent or who were using a nucleoside analogue were also excluded.
Study design and treatment
The ATTAX3 study was a randomised, phase II, open-label, multicentre study of docetaxel-based chemotherapy with or without panitumumab. Patients received docetaxel 30 mg m−2 d1 and d8, cisplatin 60 mg m−2 d1 and a fluoropyrimidine (investigator choice of 5-fluorouracil infusion 160 mg m−2 per day or capecitabine 500 mg m−2 b.d. continuous) alone or with panitumumab 9 mg kg−1 d1 q3w. Treatment was administered for 8 cycles or until the patient or physician requested cessation, or until disease progression was documented. In the absence of progression or significant toxicity, panitumumab could be continued beyond 8 cycles at the discretion of the investigator.
Evaluation and outcomes
Patients had clinical assessments at baseline, before every cycle of treatment, at the end of study treatment and then every 12 weeks. Patients were assessed radiologically at baseline, 6 weekly during the trial and then every 12 weeks until disease progression. Toxicity was graded using NCI CTCAE version 3.0.
Quality of life was assessed at baseline, every 3 weeks for the first 12 weeks, every 6 weeks for the next 12 weeks and then every 12 weeks until disease progression, with the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ) C30, Version 3.0 (01/02/2003), together with the oesophageal-specific module (OES 18) or the gastric module (STO 22), depending on the site of disease.
Statistical design
The primary end point was the objective tumour response, as assessed by RECIST version 1.1. Secondary end points were overall survival, progression-free survival, treatment-related toxicity and quality of life. Tumour tissue was collected for future exploratory biomarker analysis.
Enrolment was planned for 100 patients in total, with participants randomised 1 : 1 to receive chemotherapy alone or in combination with panitumumab. A response rate of 50% was considered to be of interest for further study, and 50 patients in the experimental arm would provide a 95% confidence interval that excluded 35% (the expected response rate in the chemotherapy-alone arm). Patients were randomised by the minimisation method and were stratified by primary tumour site (oesophagus, oesophagogastric junction or stomach), histology (adenocarcinoma, squamous or undifferentiated carcinoma), WHO performance status (0–1 vs 2), use of 5-fluorouracil vs capecitabine and institution.
Toxicity was evaluated by the Independent Data Monitoring Committee (IDMC) after 20 patients had been enrolled in the combination arm of the study and had completed at least 2 cycles of therapy and again after the REAL3 safety alert.
The 95% confidence intervals for response rates were calculated by the Wilson method. Time-to-event analyses were summarised with Kaplan–Meier curves. All analyses were by intention to treat. Analyses used SAS (version 9.2; Cary, NC, USA).
Results
Patient characteristics
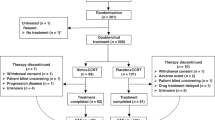
Between April 2010 and November 2011, 77 patients from 15 institutions in Australia were registered; 39 were randomised to receive chemotherapy alone and 38 to chemotherapy plus panitumumab (see consort diagram; Figure 1). All patients enrolled were eligible. One patient was randomised but did not commence treatment. Baseline characteristics were similar in the two groups (Table 1). Approximately 90% of participants had adenocarcinoma, and two-thirds received a capecitabine-containing regimen. Most patients (90.8%) had distant metastases at the time of study enrolment.
A safety alert from the REAL3 trial prompted a review of the trial data by the IDMC. This committee observed no evidence of adverse outcomes associated with panitumumab, but it was felt that it would be unlikely that the study would meet its primary end point even if it completed its accrual target. Consequently, it was recommended that new enrolments cease, but patients already enrolled continued treatment according to protocol.
Treatment
Patients in the chemotherapy-alone arm received a median of 7 cycles of treatment (range 2–16) and those in the chemotherapy plus panitumumab combination arm a median of 6 cycles of treatment (range 1–12). Four (10.5%) patients in the combination arm and two (5.1%) in the chemotherapy-alone arm discontinued therapy early (within 6 weeks). The predominant reasons for early treatment discontinuation were patient or clinician preference.
Efficacy
Of the 77 patients recruited to the study, 71 were evaluable for response. In the chemotherapy-alone arm, no patients had a complete response, 19 (48.7%) had a partial response and 17(43.6%) had stable disease. In the combination arm, 2 patients had a complete response, 20 (52.6%) a partial response and 10 (26.3%) had stable disease. Overall, 48.7% of patients in the chemotherapy-alone arm had an objective tumour response (51.4% if early withdrawals were excluded) and 57.9% in the combination arm had an objective tumour response (64.7% if early withdrawals were excluded). After a median follow-up of 24 months, median progression-free survival was 6.9 months in the chemotherapy-alone arm and 6.0 months in the combination arm (Figure 2). Median overall survival was 11.7 months in the chemotherapy arm and 10 months in the combination arm (Figure 3).
Toxicity
Across both arms of the study, the most common significant adverse events (of grade 3 or higher) were anorexia, infection, vomiting, diarrhoea and fatigue (Table 2). The rate of grade 2 or 3 infection with normal neutrophils was higher in the chemotherapy plus panitumumab arm (62.1% vs 33.3%), and 94.6% of patients in the combination arm experienced an acneiform rash of any grade (8.1% of grade 3). In the chemotherapy-alone arm, 31% (n=12) required a dose delay of at least 7 days during treatment, compared with 49% (n=18) in the combination arm.
Quality of life
Improvement in a specific disease-associated symptom or aspect of quality of life was defined as an increase of 10 points or more for that questionnaire item for more than 3 weeks. Of the participants, 95% completed at least one QOL assessment (4 patients did not participate because of insufficient English). Of these patients, 86% completed a baseline assessment and questionnaires until progression. In a combined analysis of all participants, significant improvement was seen between baseline and time of progression in pain (change from baseline, 11.36), with a trend to improvement in emotional functioning (change from baseline, 9.26). In those with gastric primary tumours who completed the QLQ stomach module, there was additionally an improvement in dysphagia and anxiety. Worsening of dry mouth and taste disturbance was significant across the combined group, with a trend to worsening of diarrhoea. Overall, global health status was stable across the trial period (change from baseline, 1.02).
Discussion
In this trial, adding panitumumab to triplet chemotherapy using docetaxel, cisplatin and a fluoropyrimidine did not lead to a significant improvement in objective tumour response rate, progression-free survival or overall survival. The median overall survival (11.7 months in the chemotherapy-alone arm and 10 months in the chemotherapy plus panitumumab arm) was consistent with results from similar studies in similar populations (Van Cutsem et al, 2006; Al-Batran et al, 2008; Lordick et al, 2013). There was a higher rate of some toxicities in the combination arm, in keeping with the known side-effect profile of panitumumab. Overall, global quality-of-life scores remained stable throughout the trial.
There is now increasing evidence that, despite promising phase II evidence suggesting a role for the targeting of the EGFR pathway as a new option for treatment of oesophagogastric cancer, this has failed to translate to significant clinical benefit in larger randomised trials.
The randomised phase III EXPAND trial (Lordick et al, 2013), using a first-line regimen of capecitabine and cisplatin with or without cetuximab for advanced oesophagogastric cancer, also failed to show a benefit from the addition of EGFR-targeted therapy in an unselected population. This is also in keeping with results from the REAL 3 trial (Waddell et al, 2013) that randomised 575 patients to receive chemotherapy with epirubicin, oxaliplatin and capecitabine alone or with panitumumab (with dose modification of the chemotherapy backbone). REAL 3 showed no benefit to the combination arm, and in fact revealed an inferior survival for those receiving the EGFR antibody. It has been postulated that this finding of a statistically inferior survival outcome in the combination arm may result from the use of modified-dose chemotherapy, or a possible negative interaction between panitumumab and components of the chemotherapy regimen. Notably, this study did not show a significantly detrimental outcome in the panitumumab arm, suggesting that the modification of chemotherapy dosing in the REAL3 study may be the factor accounting for inferior survival.
The use of a biomarker-unselected population may contribute to the lack of benefit seen across these trials. Previous work using the ATTAX2 trial (Tebbutt et al, 2013) samples available showed all tumours were wild type for KRAS exon 2/BRAF exon 15 and PI3KCA exon 20, in keeping with similar work showing low levels of mutations in this population. However, with the increasing recognition of the presence and effect of other gene abnormalities that may be amenable to direct targeting, such as EGFR and MET amplification (Lee et al, 2011), further progress in the outcomes for gastro-oesophageal cancer is likely to require better identification of biomarkers and subpopulations most likely to respond to targeted therapies.
In conclusion, the ATTAX3 trial showed there was no significant improvement in activity for the combination of docetaxel-based chemotherapy and panitumumab over chemotherapy alone in the treatment of advanced oesophagogastric cancer.
Change history
01 March 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Al-Batran S-E, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, Rethwisch V, Seipelt G, Homann N, Wilhelm G, Schuch G, Stoehlmacher J, Derigs HG, Hegewisch-Becker S, Grossmann J, Pauligk C, Atmaca A, Bokemeyer C, Knuth A, Jäger E Arbeitsgemeinschaft Internistische Onkologie (2008) Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internisticsche Onkologie. J Clin Oncol 26 (9): 1435–1442.
Cook N, Marshall A, Blazeby J, Bridgewater J, Wadsley J, Coxon F, Mansoor W, Madhusudan S, Falk S, Middleton G, Swinson D, Chau I, Thompson J, Cunningham D, Kareclas P, Dunn J, Ford H (2013) Cougar-01: a randomised phase III study of docetaxel versus active symptom control in patients with relapsed esophago-gastric adenocarcinoma. J Clin Oncol 31 (15–suppl): abstract 4023.
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358: 36–46.
Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjödén PO, Haglund U, Svensson C, Enander LK, Linné T, Sellström H, Heuman R (1997) Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 8: 163–168.
Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, Tebbutt NC, van Hazel G, Wierzbicki R, Langer C, Moore MJ (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357 (20): 2040–2048.
Langer R, Von Rahden BH, Nahrig J, Von Weyhern C, Reiter R, Feith M, Stein HJ, Siewert JR, Höfler H, Sarbia M (2006) Prognostic significance of expression patterns of c-erbB-2, p53, p16INK4A, p27KIP1, cyclin D1 and epidermal growth factor receptor in esophageal adenocarcinoma: a tissue microarray study. J Clin Pathol 59: 631–634.
Lee J, Seo JW, Jun HJ, Ki CS, Park SH, Park YS, Lim HY, Choi MG, Bae JM, Sohn TS, Noh JH, Kim S, Jang HL, Kim JY, Kim KM, Kang WK, Park JO (2011) Impact of MET amplification on gastric cancer: possible roles as a novel prognostic marker and a potential therapeutic target. Oncol Rep 25 (6): 1517–1524.
Lieto E, Ferraraccio F, Orditura M, Castellano P, Mura AL, Pinto M, Zamboli A, De Vita F, Galizia G (2008) Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol 15 (1): 69–79.
Lordick F, Kang Y-K, Chung H-C, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, Park JO, Sawaki A, Celik I, Götte H, Melezínková H, Moehler M Arbeitsgemeinschaft Internistische Onkologie and EXPAND Investigators (2013) Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 14 (6): 490–499.
Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M (1993) Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 72 (1): 37–41.
Muro K, Hamaguchi T, Ohtsu A (2004) A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol 2004 16 (6): 955–959.
Navarini D, Gurski RR, Madalosso CA, Aita L, Meurer L, Fornari F (2012) Epidermal growth factor receptor in esophageal adenocarcinoma: relationship with tumor stage and survival after esophagectomy. Gastroenterol Res Pract 2012: 941954.
Tebbutt NC, Cummins MM, Sourjina T, Strickland A, Van Hazel G, Ganju V, Gibbs D, Stockler M, Gebski V, Zalcberg J Australasian Gastro-Intestinal Trials Group (2010) Randomised, non-comparative phase II study of weekly docetaxel with cisplatin and 5-fluorouracil or with capecitabine in oesophagogastric cancer: the AGITG ATTAX trial. Br J Cancer 102 (3): 475–481.
Tebbutt NC, Parry MM, Zannino D, Strickland AH, Van Hazel GA, Pavlakis N, Ganju V, Mellor D, Dobrovic A, Gebski VJ Australasian Gastro-Intestinal Trials Group (AGITG (2013) Docetaxel plus cetuximab as second-line treatment for docetaxel-refractory oesophagogastric cancer: the AGITG ATTAX2 trial. Br J Cancer 108: 771.
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA V325 Study Group (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24 (31): 4991–4997.
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25 (13): 1658–1664.
Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J, Ferry D, Mansoor W, Crosby T, Coxon F, Smith D, Waters J, Iveson T, Falk S, Slater S, Peckitt C, Barbachano Y (2013) Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 14: 481–489.
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE (2006) Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 24 (18): 2903–2909.
Acknowledgements
Amgen Australia and Sanofi Australia provided funding to support the study. Neither company was involved in the study design, or data collection, analysis or interpretation. The content of the manuscript and the decision to submit was made independently by the authors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
Niall Tebbutt, Timothy Price and Jennifer Shannon have had consultant or advisory roles for Amgen. Niall Tebbutt has given expert testimony for and has had research funding from Amgen. All other authors have no relevant relationships to disclose.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Tebbutt, N., Price, T., Ferraro, D. et al. Panitumumab added to docetaxel, cisplatin and fluoropyrimidine in oesophagogastric cancer: ATTAX3 phase II trial. Br J Cancer 114, 505–509 (2016). https://doi.org/10.1038/bjc.2015.440
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.440
Keywords
This article is cited by
-
Comparative efficacy and tolerability of targeted and immunotherapy combined with chemotherapy as first-line treatment for advanced gastric cancer: a Bayesian network meta-analysis
Scientific Reports (2022)
-
Helicobacter pylori and gastric cancer: a lysosomal protease perspective
Gastric Cancer (2022)
-
First-line panitumumab plus docetaxel and cisplatin in advanced gastric and gastro-oesophageal junction adenocarcinoma: results of a phase II trial
Clinical and Translational Oncology (2020)
-
Reporting of health-related quality of life in randomized controlled trials involving palliative systemic therapy for esophagogastric cancer: a systematic review
Gastric Cancer (2018)
-
Skin toxicity with anti-EGFR monoclonal antibody in cancer patients: a meta-analysis of 65 randomized controlled trials
Cancer Chemotherapy and Pharmacology (2018)